In vitro infection of ovine cell lines by Jaagsiekte sheep retrovirus
- PMID: 10559321
- PMCID: PMC113058
- DOI: 10.1128/JVI.73.12.10070-10078.1999
In vitro infection of ovine cell lines by Jaagsiekte sheep retrovirus
Abstract
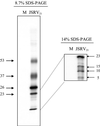
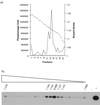
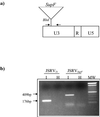
Sheep pulmonary adenomatosis (SPA), also known as jaagsiekte or ovine pulmonary carcinoma, is a contagious lung cancer of sheep, originating from type II pneumocytes and Clara cells. Previous studies have implicated a type D retrovirus (jaagsiekte sheep retrovirus [JSRV]) as the causative agent of SPA. We recently isolated a proviral clone of JSRV from an animal with a spontaneous case of SPA (JSRV(21)) and showed that it harbors an infectious and oncogenic virus. This demonstrated that JSRV is necessary and sufficient to induce SPA. A major impediment in research on JSRV has been the lack of an in vitro tissue culture system for the virus. The experiments reported here show the first successful in vitro infection with this virus, using the JSRV(21) clone. JSRV(21) virus was obtained by transiently transfecting human 293T cells with a plasmid containing the JSRV(21) provirus driven by the human cytomegalovirus immediate-early promoter. Virus produced in this manner exhibited reverse transcriptase (RT) activity that banded at 1.15 g/ml in sucrose density gradients. Infection of concentrated JSRV(21) into ovine choroid plexus (CP), testes (OAT-T3), turbinate (FLT), and intestinal carcinoma (ST6) cell lines resulted in establishment of infection as measured by PCR amplification. Evidence that this reflected genuine infection included the fact that heat inactivation of the virus eliminated it, the levels of viral DNA increased with passage of the infected cells, and the infected cells released active RT as measured by the sensitive product enhancement RT assay. The RT activity released from the infected cells banded at 1.15 g/ml, and JSRV(21) provirus was transmitted from infected cells to uninfected ones by cocultivation. However, the amount of virus released from infected cells was low. These results suggest that the JSRV receptor is present on many ovine cell types and that the observed restriction of JSRV expression in vivo to tumor cells might be controlled by factors other than the viral receptor. Finally we tagged the U3 of pJSRV(21) with the bacterial supF gene, an amber suppressor tRNA gene. The resulting clone, termed pJSRV(supF), is infectious in vitro. It may be a useful tool for future studies on viral DNA integration, since the normal sheep genome contains 15 to 20 copies of highly JSRV-related endogenous sequences that cross-react with many JSRV hybridization probes.
Figures





References
-
- Bradac J, Hunter E. Polypeptides of Mason-Pfizer monkey virus. I. Synthesis and processing of the gag-gene products. Virology. 1984;138:260–275. - PubMed
-
- DeMartini, J. C., and J. M. Sharp. Unpublished data.
-
- Dion A S, Williams C J, Pomenti A A. The major structural proteins of murine mammary tumor virus: techniques for isolation. Anal Biochem. 1977;82:18–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

