Priming with a secreted form of the fusion protein of respiratory syncytial virus (RSV) promotes interleukin-4 (IL-4) and IL-5 production but not pulmonary eosinophilia following RSV challenge
- PMID: 10559323
- PMCID: PMC113060
- DOI: 10.1128/JVI.73.12.10086-10094.1999
Priming with a secreted form of the fusion protein of respiratory syncytial virus (RSV) promotes interleukin-4 (IL-4) and IL-5 production but not pulmonary eosinophilia following RSV challenge
Abstract
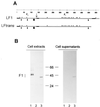
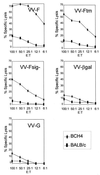
The attachment (G) protein of respiratory syncytial virus (RSV) is synthesized as two mature forms: a membrane-anchored form and a smaller secreted form. BALB/c mice scarified with vaccinia virus (VV) expressing the secreted form develop a greater pulmonary eosinophilic influx following RSV challenge than do mice scarified with VV expressing the membrane-anchored form. To determine if a soluble form of an RSV protein was sufficient to induce eosinophilia following RSV challenge, a cDNA that encoded a secreted form of the fusion (F) protein of RSV was constructed and expressed in VV (VV-Ftm(-)). Splenocytes and lung lymphocytes from mice primed with VV-Ftm(-) produced significantly more of the Th2 cytokines interleukin-4 (IL-4) and IL-5 than did mice vaccinated with VV expressing either the native (membrane-anchored) form of the F protein or the G protein. Although mice scarified with VV-Ftm(-) developed a slight increase in the number of pulmonary eosinophils following RSV infection, the increase was not as great as that seen in VV-G-primed mice. Despite the increased IL-4 and IL-5 production and in contrast to mice primed with VV-G, mice primed with VV-Ftm(-) developed RSV-specific cytotoxic T lymphocytes (CTL) and maintained high levels of gamma interferon production. These data demonstrate that recombinant VV strains expressing soluble forms of RSV proteins induce immune responses that are more Th2-like. However, this change alone does not appear sufficient to induce vaccine-augmented disease in the face of active CD8(+) CTL populations.
Figures
References
-
- Alwan W H, Openshaw P J. Distinct patterns of T- and B-cell immunity to respiratory syncytial virus induced by individual viral proteins. Vaccine. 1993;11:431–437. - PubMed
-
- Alwan W H, Record F M, Openshaw P J M. Phenotypic and functional characterisation of T cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993;150:5211–5218. - PubMed
-
- Arbiza J, Taylor G, Lopez J A, Furze J, Wyld S, Whyte P, Stott E J, Wertz G, Sullender W, Trudel M, et al. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1992;73:2225–2234. - PubMed
-
- Bembridge G P, Garcia Beato R, Lopez J A, Melero J A, Taylor G. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. J Immunol. 1998;161:2473–2480. - PubMed
-
- Bembridge G P, Lopez J A, Cook R, Melero J A, Taylor G. Recombinant vaccinia virus coexpressing the F protein of respiratory syncytial virus (RSV) and interleukin-4 (IL-4) does not inhibit the development of RSV-specific memory cytotoxic T lymphocytes, whereas priming is diminished in the presence of high levels of IL-2 or gamma interferon. J Virol. 1998;72:4080–4087. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

