Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1
- PMID: 10559354
- PMCID: PMC113091
- DOI: 10.1128/JVI.73.12.10359-10370.1999
Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1
Abstract
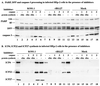
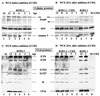
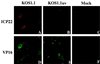
Cultured human epithelial cells infected with an ICP27 deletion strain of herpes simplex virus type 1 (HSV-1) show characteristic features of apoptotic cells including cell shrinkage, nuclear condensation, and DNA fragmentation. These cells do not show such apoptotic features when infected with a wild-type virus unless the infections are performed in the presence of a protein synthesis inhibitor. Thus, both types of virus induce apoptosis, but the ICP27-null virus is unable to prevent this process from killing the cells. In this report, we show that this ICP27-deficient virus induced apoptosis in human HEp-2 cells through a pathway which involved the activation of caspase-3 and the processing of the death substrates DNA fragmentation factor and poly(ADP-ribose) polymerase. The induction of apoptosis by wild-type HSV-1 occurred prior to 6 h postinfection (hpi), and de novo viral protein synthesis was not required to induce the process. The ability of the virus to inhibit apoptosis was shown to be effective between 3 to 6 hpi. Wild-type HSV-1 infection was also able to block the apoptosis induced in cells by the addition of cycloheximide, staurosporine, and sorbitol. While U(S)3- and ICP22-deficient viruses showed a partial prevention of apoptosis, deletion of either the U(L)13 or vhs gene products did not affect the ability of HSV-1 to prevent apoptosis in infected cells. Finally, we demonstrate that in UV-inactivated viruses, viral binding and entry were not sufficient to induce apoptosis. Taken together, these results suggest that either gene expression or another RNA metabolic event likely plays a role in the induction of apoptosis in HSV-1-infected human cells.
Figures




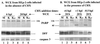

References
-
- Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. . (Letter.) - PubMed
-
- Asano S, Honda T, Goshima F, Watanabe D, Miyake Y, Sugiura Y, Nishiyama Y. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J Gen Virol. 1999;80:51–56. - PubMed
-
- Aubert, M., and J. A. Blaho. Unpublished results.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

