A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low-frequency stimulation at hippocampal CA1 synapses
- PMID: 10559382
- PMCID: PMC6782980
- DOI: 10.1523/JNEUROSCI.19-22-09728.1999
A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low-frequency stimulation at hippocampal CA1 synapses
Abstract
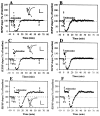
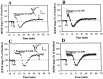
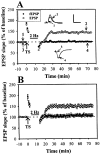
The involvement of adenosine on the development of time-dependent reversal of long-term potentiation (LTP) by low-frequency stimulation (LFS) was investigated at Schaffer collateral-CA1 synapses of rat hippocampal slices. A train of LFS (2 Hz, 10 min, 1200 pulses) had no long-term effects on synaptic transmission but produced lasting depression of previously potentiated responses. This reversal of LTP (depotentiation) was observed when the stimulus was delivered </=3 min after induction of LTP. However, application at 10 min after induction had no detectable effect on potentiation. This time-dependent reversal of LTP by LFS appeared to be mediated by extracellular adenosine, because it was mimicked by bath-applied adenosine and was specifically inhibited by the selective A(1) adenosine receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (100 nM). The effect of adenosine could be mimicked by 5-HT(1A) receptor agonist buspirone, but the LFS-induced depotentiation could not be antagonized by 5-HT(1A) receptor antagonist NAN-190. The source of extracellular adenosine in response to LFS appeared to be attributable to the efflux of cAMP. In addition, this LFS-induced depotentiation was blocked by bath application of adenylyl cyclase activator forskolin or injection of a cAMP analog Sp-adenosine cAMP (10 mM) into postsynaptic neurons. Moreover, the selective protein phosphatase 1 and 2A inhibitors okadaic acid and calyculin A prevented the LFS-induced depotentiation. These results thus suggest that increasing extracellular adenosine appears to underlie the LFS-induced depotentiation via acting on the A(1) receptor subtype to interrupt the cAMP-dependent biochemical processes leading to the LTP expression.
Figures





Similar articles
-
Masking of forskolin-induced long-term potentiation by adenosine accumulation in area CA1 of the rat hippocampus.Neuroscience. 1999 Jan;88(1):69-78. doi: 10.1016/s0306-4522(98)00200-0. Neuroscience. 1999. PMID: 10051190
-
Time-dependent reversal of long-term potentiation by low-frequency stimulation at the hippocampal mossy fiber-CA3 synapses.J Neurosci. 2001 Jun 1;21(11):3705-14. doi: 10.1523/JNEUROSCI.21-11-03705.2001. J Neurosci. 2001. PMID: 11356857 Free PMC article.
-
Prior short-term synaptic disinhibition facilitates long-term potentiation and suppresses long-term depression at CA1 hippocampal synapses.Eur J Neurosci. 1999 Nov;11(11):4059-69. doi: 10.1046/j.1460-9568.1999.00819.x. Eur J Neurosci. 1999. PMID: 10583494
-
Progress in understanding the factors regulating reversibility of long-term potentiation.Rev Neurosci. 2001;12(1):51-68. doi: 10.1515/revneuro.2001.12.1.51. Rev Neurosci. 2001. PMID: 11236065 Review.
-
Involvement of postsynaptic G-proteins in hippocampal long-term potentiation.Rev Neurosci. 1994 Jan-Mar;5(1):1-9. doi: 10.1515/revneuro.1994.5.1.1. Rev Neurosci. 1994. PMID: 8019702 Review.
Cited by
-
Local protein synthesis and GABAB receptors regulate the reversibility of long-term potentiation at murine hippocampal mossy fibre-CA3 synapses.J Physiol. 2004 Nov 15;561(Pt 1):91-108. doi: 10.1113/jphysiol.2004.072546. Epub 2004 Sep 2. J Physiol. 2004. PMID: 15345751 Free PMC article.
-
Depotentiation of Long-Term Potentiation Is Associated with Epitope-Specific Tau Hyper-/Hypophosphorylation in the Hippocampus of Adult Rats.J Mol Neurosci. 2019 Feb;67(2):193-203. doi: 10.1007/s12031-018-1224-x. Epub 2018 Nov 29. J Mol Neurosci. 2019. PMID: 30498986
-
Cannabinoids in Parkinson's Disease.Cannabis Cannabinoid Res. 2017 Feb 1;2(1):21-29. doi: 10.1089/can.2017.0002. eCollection 2017. Cannabis Cannabinoid Res. 2017. PMID: 28861502 Free PMC article. Review.
-
Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits.Proc Natl Acad Sci U S A. 2012 Apr 24;109(17):6710-5. doi: 10.1073/pnas.1121147109. Epub 2012 Apr 9. Proc Natl Acad Sci U S A. 2012. PMID: 22493252 Free PMC article.
-
Separate Ionotropic and Metabotropic Glutamate Receptor Functions in Depotentiation vs. LTP: A Distinct Role for Group1 mGluR Subtypes and NMDARs.Front Cell Neurosci. 2016 Nov 7;10:252. doi: 10.3389/fncel.2016.00252. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27872582 Free PMC article.
References
-
- Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:1–11. - PubMed
-
- Arai A, Kessler M, Lynch G. The effects of adenosine on the development of long-term potentiation. Neurosci Lett. 1990;19:41–44. - PubMed
-
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
