Embryonic neurons adapt to the inhibitory proteoglycan aggrecan by increasing integrin expression
- PMID: 10559411
- PMCID: PMC6782993
- DOI: 10.1523/JNEUROSCI.19-22-10036.1999
Embryonic neurons adapt to the inhibitory proteoglycan aggrecan by increasing integrin expression
Abstract
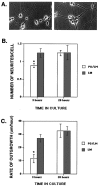
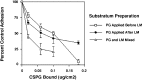
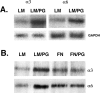
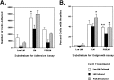
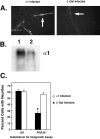
The primary mediators of cell migration during development, wound healing and metastasis, are receptors of the integrin family. In the developing and regenerating nervous system, chondroitin sulfate proteoglycans (CSPGs) inhibit the integrin-dependent migration of neuronal growth cones. Here we report that embryonic sensory neurons cultured on the growth-promoting molecule laminin in combination with the inhibitory CSPG aggrecan rapidly adapt to inhibition. Adaptation is associated with a two- to threefold increase in the levels of RNA and surface protein for two laminin receptors, integrin alpha6beta1 and alpha3beta1, indicating that integrin expression is regulated by aggrecan. Increased integrin expression is associated both with increases in neuronal cell adhesion/outgrowth and with decreases in the ability of aggrecan to inhibit cell adhesion. Directly increasing integrin expression by adenoviral infection is sufficient to eliminate the inhibitory effects of aggrecan, indicating that upregulation of integrin receptors may promote neuronal regeneration in the presence of inhibitory matrix components.
Figures
References
-
- Barkalow FJ, Schwarzbauer JE. Interactions between fibronectin and chondroitin sulfate are modulated by molecular context. J Biol Chem. 1994;269:3957–3962. - PubMed
-
- Barnea G, Grumet M, Milev P, Silvennoinen O, Levy JB, Sap J, Schlessinger J. Receptor tyrosine phosphatase beta is expressed in the form of proteoglycan and binds to the extracellular matrix protein tenascin. J Biol Chem. 1994;269:14349–14352. - PubMed
-
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. - PubMed
-
- Braunewell KH, Pesheva P, McCarthy JB, Furcht LT, Schmitz B, Schachner M. Functional involvement of sciatic nerve-derived versican- and decorin-like molecules and other chondroitin sulphate proteoglycans in ECM-mediated cell adhesion and neurite outgrowth. Eur J Neurosci. 1995;7:805–814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
