Gaze direction modulates finger movement activation patterns in human cerebral cortex
- PMID: 10559412
- PMCID: PMC6782988
- DOI: 10.1523/JNEUROSCI.19-22-10044.1999
Gaze direction modulates finger movement activation patterns in human cerebral cortex
Abstract
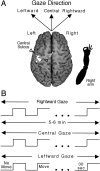
We investigated whether gaze direction modified the pattern of finger movement activation in human cerebral cortex using functional magnetic resonance imaging (MRI). Participants performed a sequential finger-tapping task or made no finger movements while maintaining gaze in the direction of the moving hand (aligned conditions) or away from the location of the moving hand. Functional MR signals, measured in the hemisphere contralateral to the moving hand, revealed finger movement-related activation in primary motor cortex, lateral and medial premotor cortex, and a wide extent of the lateral superior and inferior parietal lobules. In each area, the extent of the finger movement activation increased when static gaze was more aligned with the moving hand compared to when gaze was directed away from the moving hand. These data suggest the existence of large-scale cortical networks related to finger actions and indicate that skeletomotor processing in the cerebral cortex is consistently modified by gaze direction signals.
Figures




References
-
- Andersen RA. Coordinate transformations and motor planning in posterior parietal cortex. In: Gazzaniga M, editor. The cognitive neurosciences. MIT; Cambridge: 1995. pp. 519–532.
-
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. - PubMed
-
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. - PubMed
-
- Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science. 1999;285:257–260. - PubMed
-
- Boussaoud D. Primate premotor cortex: modulation of preparatory neuronal activity by gaze. J Neurophysiol. 1995;73:886–890. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
