Assessment of inhibition and epileptiform activity in the septal dentate gyrus of freely behaving rats during the first week after kainate treatment
- PMID: 10559413
- PMCID: PMC6782973
- DOI: 10.1523/JNEUROSCI.19-22-10053.1999
Assessment of inhibition and epileptiform activity in the septal dentate gyrus of freely behaving rats during the first week after kainate treatment
Abstract
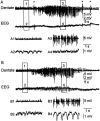
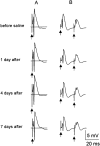
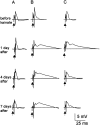
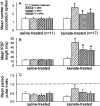
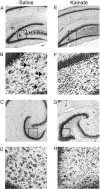
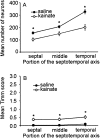
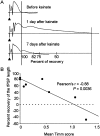
Mossy fiber reorganization has been hypothesized to restore inhibition months after kainate-induced status epilepticus. The time course of recovery of inhibition after kainate treatment, however, is not well established. We tested the hypothesis that if inhibition is decreased after kainate treatment, it is restored within the first week when little or no mossy fiber reorganization has occurred. Chronic in vivo recordings of the septal dentate gyrus were performed in rats before and 1, 4, and 7-8 d after kainate (multiple injections of 5 mg/kg, i.p.; n = 17) or saline (n = 11) treatment. Single and paired-pulse stimuli were used to assess synaptic inhibition. The first day after kainate treatment, only a fraction of rats showed multiple population spikes (35%), prolonged field postsynaptic potentials (76%), and loss of paired-pulse inhibition (29%) to perforant path stimulation. Thus, inhibition was reduced in only some of the kainate-treated rats. By 7-8 d after treatment, nearly all kainate-treated rats showed partial or full recovery in these response characteristics. Histological analysis indicated that kainate-treated rats had a significant decrease in the number of hilar neurons compared to controls, but Timm staining showed little to no mossy fiber reorganization. These results suggest that a decrease in synaptic inhibition in the septal dentate gyrus is not a prerequisite for epileptogenesis and that most of the recovery of inhibition occurs before robust Timm staining in the inner molecular layer.
Figures






Similar articles
-
Abnormal responses to perforant path stimulation in the dentate gyrus of slices from rats with kainate-induced epilepsy and mossy fiber reorganization.Epilepsy Res. 1999 Aug;36(1):31-42. doi: 10.1016/s0920-1211(99)00022-4. Epilepsy Res. 1999. PMID: 10463848
-
Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats.J Comp Neurol. 1997 Sep 1;385(3):385-404. J Comp Neurol. 1997. PMID: 9300766
-
Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization.J Neurophysiol. 1997 May;77(5):2685-96. doi: 10.1152/jn.1997.77.5.2685. J Neurophysiol. 1997. PMID: 9163384
-
Repetitive perforant-path stimulation induces epileptiform bursts in minislices of dentate gyrus from rats with kainate-induced epilepsy.J Neurophysiol. 2011 Feb;105(2):522-7. doi: 10.1152/jn.00456.2010. Epub 2010 Dec 8. J Neurophysiol. 2011. PMID: 21148094 Free PMC article.
-
Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth.J Neurophysiol. 1999 Apr;81(4):1645-60. doi: 10.1152/jn.1999.81.4.1645. J Neurophysiol. 1999. PMID: 10200201
Cited by
-
How does the balance of excitation and inhibition shift during epileptogenesis?Epilepsy Curr. 2007 May-Jun;7(3):86-8. doi: 10.1111/j.1535-7511.2007.00181.x. Epilepsy Curr. 2007. PMID: 17520084 Free PMC article. No abstract available.
-
Neural stem cell grafting in an animal model of chronic temporal lobe epilepsy.Curr Protoc Stem Cell Biol. 2011 Sep;Chapter 2:Unit2D.7. doi: 10.1002/9780470151808.sc02d07s18. Curr Protoc Stem Cell Biol. 2011. PMID: 21913169 Free PMC article.
-
Single and repetitive paired-pulse suppression: a parametric analysis and assessment of usefulness in epilepsy research.Epilepsia. 2009 Apr;50(4):904-16. doi: 10.1111/j.1528-1167.2008.01939.x. Epub 2008 Dec 15. Epilepsia. 2009. PMID: 19170733 Free PMC article.
-
Initial loss but later excess of GABAergic synapses with dentate granule cells in a rat model of temporal lobe epilepsy.J Comp Neurol. 2010 Mar 1;518(5):647-67. doi: 10.1002/cne.22235. J Comp Neurol. 2010. PMID: 20034063 Free PMC article.
-
Differential vulnerability of interneurons in the epileptic hippocampus.Front Cell Neurosci. 2013 Oct 1;7:167. doi: 10.3389/fncel.2013.00167. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24098270 Free PMC article.
References
-
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. - PubMed
-
- Bekenstein JW, Lothman EW. Dormancy of inhibitory interneurons in a model of temporal lobe epilepsy. Science. 1993;259:97–100. - PubMed
-
- Ben-Ari Y. Limbic seizures and brain damage produced by kainic acid: Mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. - PubMed
-
- Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J Neurophysiol. 1997a;77:2685–2696. - PubMed
-
- Buckmaster PS, Dudek FE. Neuron loss, granule cell reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997b;385:385–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
