Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats
- PMID: 10559423
- PMCID: PMC6782959
- DOI: 10.1523/JNEUROSCI.19-22-10153.1999
Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats
Abstract
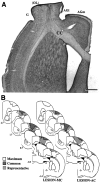
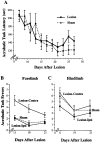
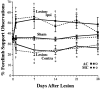
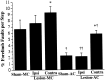
To assess behavioral experience effects on synaptic plasticity after brain damage, the present study examined the effects of complex motor skills training (the acrobatic task) on synaptic changes in layer V of the motor cortex opposite unilateral damage to the forelimb sensorimotor cortex (FLsmc). Adult male rats were given lesions or sham operations followed by 28 d of training on the acrobatic task [acrobat condition (AC)]. As a motor activity control [motor control (MC)], lesion and sham animals were given simple repetitive exercise. Previously, FLsmc lesions and acrobatic training have independently been found to result in increases in synapse to neuron ratios in the intact motor cortex relative to controls, and both of these effects were replicated in the present study. In addition, acrobat training after lesions significantly increased layer V synapses per neuron relative to sham-AC and lesion-MC rats. Thus, the combination of acrobatic training and lesions resulted in an enhanced synaptogenic response. Synapse subtypes were also differentially affected by the conditions. Lesion-MC and sham-AC primarily had increases in the number of synapses per neuron formed by multiple synaptic boutons in comparison to sham-MC. In contrast, lesion-AC had increases in both multiple and single synapses. Multiple synaptic spines and perforated synapses were also differentially affected by training versus lesions. On tests of coordinated forelimb use, lesion-AC rats performed better than lesion-MC rats. In addition to supporting a link between behavioral experience and structural plasticity after brain damage, these findings suggest that adaptive neural plasticity may be enhanced using behavioral manipulations as "therapy."
Figures



References
-
- Adams FS, Schwarting RK, Huston JP. Behavioral and neurochemical asymmetries following unilateral trephination of the rat skull: is this control operation always appropriate? Physiol Behav. 1994;55:947–952. - PubMed
-
- Anker RL, Cragg BG. Estimation of the number of synapses in a volume of nervous tissue from counts in thin sections by electron microscopy. J Neurocytol. 1974;3:725–735. - PubMed
-
- Barth TM, Jones TA, Schallert T. Functional subdivisions of the rat somatic sensorimotor cortex. Behav Brain Res. 1990;39:73–95. - PubMed
-
- Bendre AA, Swain RA, Wheeler BC, Greenough WT. Augmentation of cerebellar responses to parallel fiber activation following skilled motor acquisition in rats. Soc Neurosci Abstr. 1995;21:444.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
