The spinal muscular atrophy disease gene product, SMN: A link between snRNP biogenesis and the Cajal (coiled) body
- PMID: 10562276
- PMCID: PMC2156166
- DOI: 10.1083/jcb.147.4.715
The spinal muscular atrophy disease gene product, SMN: A link between snRNP biogenesis and the Cajal (coiled) body
Abstract
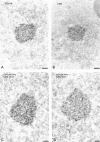
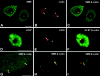
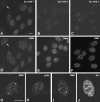
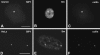
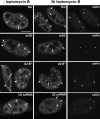
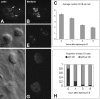
The spliceosomal snRNAs U1, U2, U4, and U5 are synthesized in the nucleus, exported to the cytoplasm to assemble with Sm proteins, and reimported to the nucleus as ribonucleoprotein particles. Recently, two novel proteins involved in biogenesis of small nuclear ribonucleoproteins (snRNPs) were identified, the Spinal muscular atrophy disease gene product (SMN) and its associated protein SIP1. It was previously reported that in HeLa cells, SMN and SIP1 form discrete foci located next to Cajal (coiled) bodies, the so-called "gemini of coiled bodies" or "gems." An intriguing feature of gems is that they do not appear to contain snRNPs. Here we show that gems are present in a variable but small proportion of rapidly proliferating cells in culture. In the vast majority of cultured cells and in all primary neurons analyzed, SMN and SIP1 colocalize precisely with snRNPs in the Cajal body. The presence of SMN and SIP1 in Cajal bodies is confirmed by immunoelectron microscopy and by microinjection of antibodies that interfere with the integrity of the structure. The association of SMN with snRNPs and coilin persists during cell division, but at the end of mitosis there is a lag period between assembly of new Cajal bodies in the nucleus and detection of SMN in these structures, suggesting that SMN is targeted to preformed Cajal bodies. Finally, treatment of cells with leptomycin B (a drug that blocks export of U snRNAs to the cytoplasm and consequently import of new snRNPs into the nucleus) is shown to deplete snRNPs (but not SMN or SIP1) from the Cajal body. This suggests that snRNPs flow through the Cajal body during their biogenesis pathway.
Figures

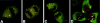


References
-
- Bertrandy S., Burlet P., Clermont O., Huber C., Fondrat C., Thierry-Mieg D., Munnich A., Lefebvre S. The RNA-binding properties of SMNdeletion analysis of the zebrafish orthologue defines domains conserved in evolution. Hum. Mol. Genet. 1999;8:775–782 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

