Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells
- PMID: 10562278
- PMCID: PMC2156170
- DOI: 10.1083/jcb.147.4.743
Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells
Erratum in
- J Cell Biol 2000 Jan 10;148(1):followi
Abstract
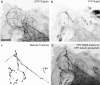
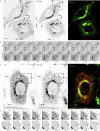
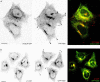
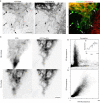
We visualized a fluorescent-protein (FP) fusion to Rab6, a Golgi-associated GTPase, in conjunction with fluorescent secretory pathway markers. FP-Rab6 defined highly dynamic transport carriers (TCs) translocating from the Golgi to the cell periphery. FP-Rab6 TCs specifically accumulated a retrograde cargo, the wild-type Shiga toxin B-fragment (STB), during STB transport from the Golgi to the endoplasmic reticulum (ER). FP-Rab6 TCs associated intimately with the ER, and STB entered the ER via specialized peripheral regions that accumulated FP-Rab6. Microinjection of antibodies that block coatomer protein I (COPI) function inhibited trafficking of a KDEL-receptor FP-fusion, but not FP-Rab6. Additionally, markers of COPI-dependent recycling were excluded from FP-Rab6/STB TCs. Overexpression of Rab6:GDP (T27N mutant) using T7 vaccinia inhibited toxicity of Shiga holotoxin, but did not alter STB transport to the Golgi or Golgi morphology. Taken together, our results indicate Rab6 regulates a novel Golgi to ER transport pathway.
Figures






References
-
- Antony C., Cibert C., Geraud G., Santa Maria A., Maro B., Mayau V., Goud B. The small GTP-binding protein rab6p is distributed from medial Golgi to the trans-Golgi network as determined by a confocal microscopic approach. J. Cell Sci. 1992;103:785–796 . - PubMed
-
- Balch W.E., McCaffery J.M., Plutner H., Farquhar M.G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852 . - PubMed
-
- Cohen A., Hannigan G.E., Williams B.R., Lingwood C.A. Roles of globotriaosyl- and galabiosylceramide in verotoxin binding and high affinity interferon receptor. J. Biol. Chem. 1987;262:17088–17091 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

