The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting
- PMID: 10562281
- PMCID: PMC2156159
- DOI: 10.1083/jcb.147.4.791
The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting
Abstract
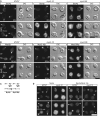
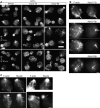
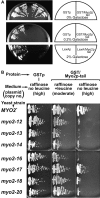
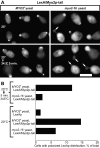
MYO2 encodes a type V myosin heavy chain needed for the targeting of vacuoles and secretory vesicles to the growing bud of yeast. Here we describe new myo2 alleles containing conditional lethal mutations in the COOH-terminal tail domain. Within 5 min of shifting to the restrictive temperature, the polarized distribution of secretory vesicles is abolished without affecting the distribution of actin or the mutant Myo2p, showing that the tail has a direct role in vesicle targeting. We also show that the actin cable-dependent translocation of Myo2p to growth sites does not require secretory vesicle cargo. Although a fusion protein containing the Myo2p tail also concentrates at growth sites, this accumulation depends on the polarized delivery of secretory vesicles, implying that the Myo2p tail binds to secretory vesicles. Most of the new mutations alter a region of the Myo2p tail conserved with vertebrate myosin Vs but divergent from Myo4p, the myosin V involved in mRNA transport, and genetic data suggest that the tail interacts with Smy1p, a kinesin homologue, and Sec4p, a vesicle-associated Rab protein. The data support a model in which the Myo2p tail tethers secretory vesicles, and the motor transports them down polarized actin cables to the site of exocytosis.
Figures




References
-
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, editors. 1996. Current Protocols in Molecular Biology. John Wiley & Sons, Inc., New York. 1,906 pp.
-
- Bailey, T., and C. Elkan. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. AAAI Press, Menlo Park, CA. 28–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

