The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol
- PMID: 10562282
- PMCID: PMC2156156
- DOI: 10.1083/jcb.147.4.809
The pro-apoptotic proteins, Bid and Bax, cause a limited permeabilization of the mitochondrial outer membrane that is enhanced by cytosol
Abstract
During apoptosis, an important pathway leading to caspase activation involves the release of cytochrome c from the intermembrane space of mitochondria. Using a cell-free system based on Xenopus egg extracts, we examined changes in the outer mitochondrial membrane accompanying cytochrome c efflux. The pro-apoptotic proteins, Bid and Bax, as well as factors present in Xenopus egg cytosol, each induced cytochrome c release when incubated with isolated mitochondria. These factors caused a permeabilization of the outer membrane that allowed the corelease of multiple intermembrane space proteins: cytochrome c, adenylate kinase and sulfite oxidase. The efflux process is thus nonspecific. None of the cytochrome c-releasing factors caused detectable mitochondrial swelling, arguing that matrix swelling is not required for outer membrane permeability in this system. Bid and Bax caused complete release of cytochrome c but only a limited permeabilization of the outer membrane, as measured by the accessibility of inner membrane-associated respiratory complexes III and IV to exogenously added cytochrome c. However, outer membrane permeability was strikingly increased by a macromolecular cytosolic factor, termed PEF (permeability enhancing factor). We hypothesize that PEF activity could help determine whether cells can recover from mitochondrial cytochrome c release.
Figures

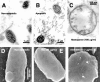
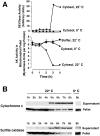
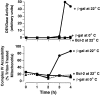


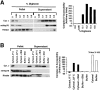

Similar articles
-
Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release.J Cell Biol. 2000 Sep 4;150(5):1027-36. doi: 10.1083/jcb.150.5.1027. J Cell Biol. 2000. PMID: 10973993 Free PMC article.
-
Inhibition of Bax-induced cytochrome c release from neural cell and brain mitochondria by dibucaine and propranolol.J Neurosci. 2003 Apr 1;23(7):2735-43. doi: 10.1523/JNEUROSCI.23-07-02735.2003. J Neurosci. 2003. PMID: 12684459 Free PMC article.
-
Bid-induced cytochrome c release is mediated by a pathway independent of mitochondrial permeability transition pore and Bax.J Biol Chem. 2000 Dec 15;275(50):39474-81. doi: 10.1074/jbc.M003370200. J Biol Chem. 2000. PMID: 10982793
-
Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis.Curr Opin Cell Biol. 2000 Aug;12(4):414-9. doi: 10.1016/s0955-0674(00)00110-1. Curr Opin Cell Biol. 2000. PMID: 10873816 Review.
-
Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c.Cell Death Differ. 2000 Dec;7(12):1166-73. doi: 10.1038/sj.cdd.4400783. Cell Death Differ. 2000. PMID: 11175253 Review.
Cited by
-
Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization.Cell. 2010 Sep 17;142(6):889-901. doi: 10.1016/j.cell.2010.08.017. Cell. 2010. PMID: 20850011 Free PMC article.
-
Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity.Mol Cell Biol. 2001 Nov;21(21):7268-76. doi: 10.1128/MCB.21.21.7268-7276.2001. Mol Cell Biol. 2001. PMID: 11585909 Free PMC article.
-
Mitochondrial permeabilization relies on BH3 ligands engaging multiple prosurvival Bcl-2 relatives, not Bak.J Cell Biol. 2007 Apr 23;177(2):277-87. doi: 10.1083/jcb.200606065. J Cell Biol. 2007. PMID: 17452531 Free PMC article.
-
Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis.J Cell Biol. 2003 Jan 6;160(1):65-75. doi: 10.1083/jcb.200208089. Epub 2003 Jan 6. J Cell Biol. 2003. PMID: 12515825 Free PMC article.
-
Disordered clusters of Bak dimers rupture mitochondria during apoptosis.Elife. 2017 Feb 6;6:e19944. doi: 10.7554/eLife.19944. Elife. 2017. PMID: 28182867 Free PMC article.
References
-
- Allen T.D., Goldberg M.W. High-resolution SEM in cell biology. Trends. Cell Biol. 1993;3:205–208 . - PubMed
-
- Allen T.D., Bennion G.R., Rutherford S.A., Reipert S., Ramalho A., Kiseleva E., Goldberg M.W. Accessing nuclear structure for field emission, in lens, scanning electron microscopy (FEISEM) Scanning Microsc. Suppl. 1996;10:149–163 . - PubMed
-
- Antonsson B., Conti F., Ciavatta A., Montessuit S., Lewis S., Martinou I., Bernasconi L., Bernard A., Mermod J.J., Mazzei G. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372 . - PubMed
-
- Barber M.J., Neame P.J. A conserved cysteine in molybdenum oxotransferases. J. Biol. Chem. 1990;265:20912–20915 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

