PAK-family kinases regulate cell and actin polarization throughout the cell cycle of Saccharomyces cerevisiae
- PMID: 10562285
- PMCID: PMC2156167
- DOI: 10.1083/jcb.147.4.845
PAK-family kinases regulate cell and actin polarization throughout the cell cycle of Saccharomyces cerevisiae
Abstract
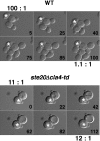
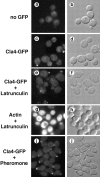
During the cell cycle of the yeast Saccharomyces cerevisiae, the actin cytoskeleton and cell surface growth are polarized, mediating bud emergence, bud growth, and cytokinesis. We have determined whether p21-activated kinase (PAK)-family kinases regulate cell and actin polarization at one or several points during the yeast cell cycle. Inactivation of the PAK homologues Ste20 and Cla4 at various points in the cell cycle resulted in loss of cell and actin cytoskeletal polarity, but not in depolymerization of F-actin. Loss of PAK function in G1 depolarized the cortical actin cytoskeleton and blocked bud emergence, but allowed isotropic growth and led to defects in septin assembly, indicating that PAKs are effectors of the Rho-guanosine triphosphatase Cdc42. PAK inactivation in S/G2 resulted in depolarized growth of the mother and bud and a loss of actin polarity. Loss of PAK function in mitosis caused a defect in cytokinesis and a failure to polarize the cortical actin cytoskeleton to the mother-bud neck. Cla4-green fluorescent protein localized to sites where the cortical actin cytoskeleton and cell surface growth are polarized, independently of an intact actin cytoskeleton. Thus, PAK family kinases are primary regulators of cell and actin cytoskeletal polarity throughout most or all of the yeast cell cycle. PAK-family kinases in higher organisms may have similar functions.
Figures
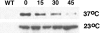
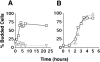



Similar articles
-
Actin cytoskeleton organization regulated by the PAK family of protein kinases.Curr Biol. 1998 Aug 27;8(17):967-70. doi: 10.1016/s0960-9822(98)00398-4. Curr Biol. 1998. PMID: 9742399
-
Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast.Mol Cell. 2000 Nov;6(5):1155-67. doi: 10.1016/s1097-2765(00)00113-1. Mol Cell. 2000. PMID: 11106754
-
Control of Lte1 localization by cell polarity determinants and Cdc14.Curr Biol. 2002 Dec 23;12(24):2098-110. doi: 10.1016/s0960-9822(02)01388-x. Curr Biol. 2002. PMID: 12498684
-
Ras-related GTPases and the cytoskeleton.Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
-
Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states.J Cell Sci. 2000 Feb;113 ( Pt 3):365-75. doi: 10.1242/jcs.113.3.365. J Cell Sci. 2000. PMID: 10639324 Review.
Cited by
-
How cells determine the number of polarity sites.Elife. 2021 Apr 26;10:e58768. doi: 10.7554/eLife.58768. Elife. 2021. PMID: 33899733 Free PMC article.
-
Genetic interactions among regulators of septin organization.Eukaryot Cell. 2004 Aug;3(4):847-54. doi: 10.1128/EC.3.4.847-854.2004. Eukaryot Cell. 2004. PMID: 15302817 Free PMC article.
-
Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway.EMBO J. 2006 Jul 12;25(13):3033-44. doi: 10.1038/sj.emboj.7601192. Epub 2006 Jun 15. EMBO J. 2006. PMID: 16778768 Free PMC article.
-
Cla4A, a Novel Regulator of Gene Expression Networks Required for Asexual and Insect-Pathogenic Lifecycles of Beauveria bassiana.Int J Mol Sci. 2024 Jun 10;25(12):6410. doi: 10.3390/ijms25126410. Int J Mol Sci. 2024. PMID: 38928117 Free PMC article.
-
Septins: molecular partitioning and the generation of cellular asymmetry.Cell Div. 2009 Aug 26;4:18. doi: 10.1186/1747-1028-4-18. Cell Div. 2009. PMID: 19709431 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

