Manner of interaction of heterogeneous claudin species within and between tight junction strands
- PMID: 10562289
- PMCID: PMC2156154
- DOI: 10.1083/jcb.147.4.891
Manner of interaction of heterogeneous claudin species within and between tight junction strands
Abstract
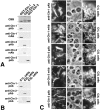
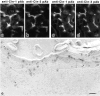
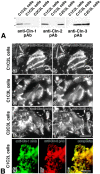
In tight junctions (TJs), TJ strands are associated laterally with those of adjacent cells to form paired strands to eliminate the extracellular space. Claudin-1 and -2, integral membrane proteins of TJs, reconstitute paired TJ strands when transfected into L fibroblasts. Claudins comprise a multigene family and more than two distinct claudins are coexpressed in single cells, raising the questions of whether heterogeneous claudins form heteromeric TJ strands and whether claudins interact between each of the paired strands in a heterophilic manner. To answer these questions, we cotransfected two of claudin-1, -2, and -3 into L cells, and detected their coconcentration at cell-cell borders as elaborate networks. Immunoreplica EM confirmed that distinct claudins were coincorporated into individual TJ strands. Next, two L transfectants singly expressing claudin-1, -2, or -3 were cocultured and we found that claudin-3 strands laterally associated with claudin-1 and -2 strands to form paired strands, whereas claudin-1 strands did not interact with claudin-2 strands. We concluded that distinct species of claudins can interact within and between TJ strands, except in some combinations. This mode of assembly of claudins could increase the diversity of the structure and functions of TJ strands.
Figures




Similar articles
-
Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier.J Cell Biol. 1999 Oct 4;147(1):195-204. doi: 10.1083/jcb.147.1.195. J Cell Biol. 1999. PMID: 10508866 Free PMC article.
-
A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts.J Cell Biol. 1998 Oct 19;143(2):391-401. doi: 10.1083/jcb.143.2.391. J Cell Biol. 1998. PMID: 9786950 Free PMC article.
-
Dynamic behavior of paired claudin strands within apposing plasma membranes.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3971-6. doi: 10.1073/pnas.0630649100. Epub 2003 Mar 21. Proc Natl Acad Sci U S A. 2003. PMID: 12651952 Free PMC article.
-
Occludin and claudins in tight-junction strands: leading or supporting players?Trends Cell Biol. 1999 Jul;9(7):268-73. doi: 10.1016/s0962-8924(99)01578-0. Trends Cell Biol. 1999. PMID: 10370242 Review.
-
The structure and function of claudins, cell adhesion molecules at tight junctions.Ann N Y Acad Sci. 2000;915:129-35. doi: 10.1111/j.1749-6632.2000.tb05235.x. Ann N Y Acad Sci. 2000. PMID: 11193568 Review.
Cited by
-
The expression patterns of tight junction protein claudin-1, -3, and -4 in human gastric neoplasms and adjacent non-neoplastic tissues.Int J Clin Exp Pathol. 2015 Jan 1;8(1):881-7. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 25755790 Free PMC article.
-
Remodeling of Tight Junctions and Enhancement of Barrier Integrity of the CACO-2 Intestinal Epithelial Cell Layer by Micronutrients.PLoS One. 2015 Jul 30;10(7):e0133926. doi: 10.1371/journal.pone.0133926. eCollection 2015. PLoS One. 2015. PMID: 26226276 Free PMC article.
-
Transporters at CNS barrier sites: obstacles or opportunities for drug delivery?Curr Pharm Des. 2014;20(10):1422-49. doi: 10.2174/13816128113199990463. Curr Pharm Des. 2014. PMID: 23789948 Free PMC article. Review.
-
Temporary opening of the blood-brain barrier with the nitrone compound OKN-007.Am J Nucl Med Mol Imaging. 2021 Oct 15;11(5):363-373. eCollection 2021. Am J Nucl Med Mol Imaging. 2021. PMID: 34754607 Free PMC article.
-
Morphologic determinant of tight junctions revealed by claudin-3 structures.Nat Commun. 2019 Feb 18;10(1):816. doi: 10.1038/s41467-019-08760-7. Nat Commun. 2019. PMID: 30778075 Free PMC article.
References
-
- Anderson J.M., van Itallie C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475 . - PubMed
-
- Balda M.S., Whitney J.A., Flores C., González S., Cereijido M., Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical–basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996;134:1031–1049 . - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

