A murine model of hereditary hemorrhagic telangiectasia
- PMID: 10562296
- PMCID: PMC409846
- DOI: 10.1172/JCI8088
A murine model of hereditary hemorrhagic telangiectasia
Abstract
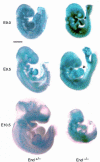
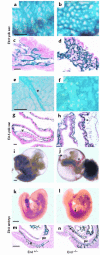
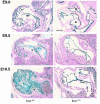
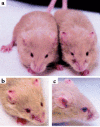
Endoglin (CD105), an accessory protein of the TGF-beta receptor superfamily, is highly expressed on endothelial cells. Hereditary hemorrhagic telangiectasia type 1 (HHT1) is associated with mutations in the Endoglin gene, leading to haploinsufficiency. To generate a disease model and ascertain the role of endoglin in development, we generated mice lacking 1 or both copies of the gene. Endoglin null embryos die at gestational day 10.0-10.5 due to defects in vessel and heart development. Vessel formation appears normal until hemorrhage occurs in yolk sacs and embryos. The primitive vascular plexus of the yolk sac fails to mature into defined vessels, and vascular channels dilate and rupture. Internal bleeding is seen in the peritoneal cavity, implying fragile vessels. Heart development is arrested at day 9.0, and the atrioventricular canal endocardium fails to undergo mesenchymal transformation and cushion-tissue formation. These data suggest that endoglin is critical for both angiogenesis and heart valve formation. Some heterozygotes, either with an inbred 129/Ola or mixed C57BL/6-129/Ola background, show signs of HHT, such as telangiectases or recurrent nosebleeds. In this murine model of HHT, it appears that epigenetic factors and modifier genes, some of which are present in 129/Ola, contribute to disease heterogeneity.
Figures

Comment in
-
Supermodels and disease: insights from the HHT mice.J Clin Invest. 1999 Nov;104(10):1335-6. doi: 10.1172/JCI8730. J Clin Invest. 1999. PMID: 10562293 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

