A PDZ-interacting domain in CFTR is an apical membrane polarization signal
- PMID: 10562297
- PMCID: PMC409842
- DOI: 10.1172/JCI7453
A PDZ-interacting domain in CFTR is an apical membrane polarization signal
Abstract
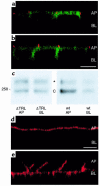
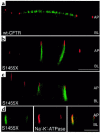
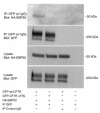
Polarization of the cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-activated chloride channel, to the apical plasma membrane of epithelial cells is critical for vectorial transport of chloride in a variety of epithelia, including the airway, pancreas, intestine, and kidney. However, the motifs that localize CFTR to the apical membrane are unknown. We report that the last 3 amino acids in the COOH-terminus of CFTR (T-R-L) comprise a PDZ-interacting domain that is required for the polarization of CFTR to the apical plasma membrane in human airway and kidney epithelial cells. In addition, the CFTR mutant, S1455X, which lacks the 26 COOH-terminal amino acids, including the PDZ-interacting domain, is mispolarized to the lateral membrane. We also demonstrate that CFTR binds to ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50), an apical membrane PDZ domain-containing protein. We propose that COOH-terminal deletions of CFTR, which represent about 10% of CFTR mutations, result in defective vectorial chloride transport, partly by altering the polarized distribution of CFTR in epithelial cells. Moreover, our data demonstrate that PDZ-interacting domains and PDZ domain-containing proteins play a key role in the apical polarization of ion channels in epithelial cells.
Figures

Similar articles
-
The PDZ-interacting domain of cystic fibrosis transmembrane conductance regulator is required for functional expression in the apical plasma membrane.J Biol Chem. 2000 Sep 1;275(35):27069-74. doi: 10.1074/jbc.M004951200. J Biol Chem. 2000. PMID: 10852925
-
Yes-associated protein 65 localizes p62(c-Yes) to the apical compartment of airway epithelia by association with EBP50.J Cell Biol. 1999 Nov 15;147(4):879-90. doi: 10.1083/jcb.147.4.879. J Cell Biol. 1999. PMID: 10562288 Free PMC article.
-
The role of the C terminus and Na+/H+ exchanger regulatory factor in the functional expression of cystic fibrosis transmembrane conductance regulator in nonpolarized cells and epithelia.J Biol Chem. 2003 Jun 13;278(24):22079-89. doi: 10.1074/jbc.M301030200. Epub 2003 Mar 21. J Biol Chem. 2003. PMID: 12651858
-
The cystic fibrosis transmembrane regulator forms macromolecular complexes with PDZ domain scaffold proteins.Proc Am Thorac Soc. 2004;1(1):28-32. doi: 10.1513/pats.2306011. Proc Am Thorac Soc. 2004. PMID: 16113408 Review.
-
Regulated trafficking of the CFTR chloride channel.Eur J Cell Biol. 2000 Aug;79(8):544-56. doi: 10.1078/0171-9335-00078. Eur J Cell Biol. 2000. PMID: 11001491 Review.
Cited by
-
The cystic fibrosis transmembrane conductance regulator controls biliary epithelial inflammation and permeability by regulating Src tyrosine kinase activity.Hepatology. 2016 Dec;64(6):2118-2134. doi: 10.1002/hep.28817. Epub 2016 Oct 27. Hepatology. 2016. PMID: 27629435 Free PMC article.
-
Characterization of polarized expression of point- or deletion-mutated human BCRP/ABCG2 in LLC-PK1 cells.Pharm Res. 2005 Mar;22(3):458-64. doi: 10.1007/s11095-004-1884-9. Pharm Res. 2005. PMID: 15835752
-
MicroRNAs and Long Non-coding RNAs in Genetic Diseases.Mol Diagn Ther. 2019 Apr;23(2):155-171. doi: 10.1007/s40291-018-0380-6. Mol Diagn Ther. 2019. PMID: 30610665 Free PMC article. Review.
-
Increases in cytosolic Ca2+ induce dynamin- and calcineurin-dependent internalisation of CFTR.Cell Mol Life Sci. 2019 Mar;76(5):977-994. doi: 10.1007/s00018-018-2989-3. Epub 2018 Dec 13. Cell Mol Life Sci. 2019. PMID: 30547226 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator-emerging regulator of cancer.Cell Mol Life Sci. 2018 May;75(10):1737-1756. doi: 10.1007/s00018-018-2755-6. Epub 2018 Feb 6. Cell Mol Life Sci. 2018. PMID: 29411041 Free PMC article. Review.
References
-
- Stanton BA. Cystic fibrosis transmembrane conductance regulator (CFTR) and renal function. Wien Klin Wochenschr. 1997;109:457–464. - PubMed
-
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. - PubMed
-
- Mickle JE, Cutting RG. Clinical implications of cystic fibrosis transmembrane conductance regulator mutations. Clin Chest Med. 1998;19:443–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

