Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells
- PMID: 10562299
- PMCID: PMC409835
- DOI: 10.1172/JCI6097
Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells
Abstract
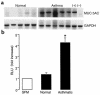
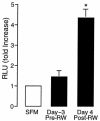
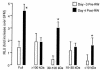
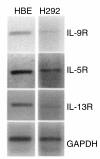
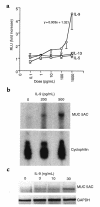
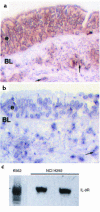
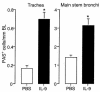
A hallmark of asthma is mucin overproduction, a condition that contributes to airway obstruction. The events responsible for mucin overproduction are not known but are thought to be associated with mediators of chronic inflammation. Others have shown that T-helper 2 (Th2) lymphocytes are required for mucous cell metaplasia, which then leads to mucin overproduction in animal models of allergy. We hypothesized that Th2 cell mediators are present in asthmatic airway fluid and directly stimulate mucin synthesis in airway epithelial cells. Results in cultured airway epithelial cells showed that samples of asthmatic fluid stimulated mucin (MUC5AC) synthesis severalfold more potently than non-asthmatic fluid. Consistent with this, lavage fluid from the airways of allergen-challenged dogs stimulated mucin synthesis severalfold more potently than that from non-allergen-challenged dogs. Fractionation of dog samples revealed 2 active fractions at <10 kDa and 30-100 kDa. Th2 cytokines in these molecular weight ranges are IL-9 (36 kDa), IL-5 (56 kDa), and IL-13 (10 kDa). Antibody blockade of ligand-receptor interaction for IL-9 (but not IL-5 or IL-13) inhibited mucin stimulation by dog airway fluid. Furthermore, recombinant IL-9, but not IL-5 or IL-13, stimulated mucin synthesis. These results indicate that IL-9 may account for as much as 50-60% of the mucin-stimulating activity of lung fluids in allergic airway disease.
Figures


References
-
- Fahy JV, Steiger DJ, Liu J, Basbaum CB, Finkbeiner WE, Boushey HA. Markers of mucus secretion and DNA levels in induced sputum from asthmatic and from health subjects. Am Rev Respir Dis. 1993;147:1132–1137. - PubMed
-
- Thurlbeck WM. Small airways disease. Hum Pathol. 1973;4:150–152. - PubMed
-
- Takizawa T, Thurlbeck W. Muscle and mucous gland size in the major bronchi of patients with chronic bronchitis, asthma and asthmatic bronchitis. Am Rev Respir Dis. 1971;104:331–336. - PubMed
-
- Reid L. Pathology of chronic bronchitis. Lancet. 1954;1:275–278. - PubMed
-
- Sperber K, Goswami SK, Gollub E, Mayer L, Marom Z. Mucus secretagogue production by a human macrophage hybridoma. J Allergy Clin Immunol. 1991;87:490–498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

