Mutation causing congenital myasthenia reveals acetylcholine receptor beta/delta subunit interaction essential for assembly
- PMID: 10562302
- PMCID: PMC409847
- DOI: 10.1172/JCI8179
Mutation causing congenital myasthenia reveals acetylcholine receptor beta/delta subunit interaction essential for assembly
Abstract
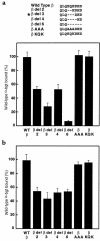
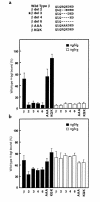
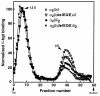
We describe a severe postsynaptic congenital myasthenic syndrome with marked endplate acetylcholine receptor (AChR) deficiency caused by 2 heteroallelic mutations in the beta subunit gene. One mutation causes skipping of exon 8, truncating the beta subunit before its M1 transmembrane domain, and abolishing surface expression of pentameric AChR. The other mutation, a 3-codon deletion (beta426delEQE) in the long cytoplasmic loop between the M3 and M4 domains, curtails but does not abolish expression. By coexpressing beta426delEQE with combinations of wild-type subunits in 293 HEK cells, we demonstrate that beta426delEQE impairs AChR assembly by disrupting a specific interaction between beta and delta subunits. Studies with related deletion and missense mutants indicate that secondary structure in this region of the beta subunit is crucial for interaction with the delta subunit. The findings imply that the mutated residues are positioned at the interface between beta and delta subunits and demonstrate contribution of this local region of the long cytoplasmic loop to AChR assembly.
Figures


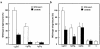
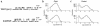
Similar articles
-
Congenital myasthenic syndromes due to heteroallelic nonsense/missense mutations in the acetylcholine receptor epsilon subunit gene: identification and functional characterization of six new mutations.Hum Mol Genet. 1997 May;6(5):753-66. doi: 10.1093/hmg/6.5.753. Hum Mol Genet. 1997. PMID: 9158150
-
Congenital myasthenic syndrome caused by low-expressor fast-channel AChR delta subunit mutation.Neurology. 2002 Dec 24;59(12):1881-8. doi: 10.1212/01.wnl.0000042422.87384.2f. Neurology. 2002. PMID: 12499478
-
End-plate acetylcholine receptor deficiency due to nonsense mutations in the epsilon subunit.Ann Neurol. 1996 Nov;40(5):810-7. doi: 10.1002/ana.410400521. Ann Neurol. 1996. PMID: 8957026
-
Congenital myasthenic syndromes: experiments of nature.J Physiol Paris. 1998 Apr;92(2):113-7. doi: 10.1016/S0928-4257(98)80147-2. J Physiol Paris. 1998. PMID: 9782453 Review.
-
Congenital myasthenic syndromes: recent advances.Arch Neurol. 1999 Feb;56(2):163-7. doi: 10.1001/archneur.56.2.163. Arch Neurol. 1999. PMID: 10025421 Review.
Cited by
-
The spectrum of congenital myasthenic syndromes.Mol Neurobiol. 2002 Oct-Dec;26(2-3):347-67. doi: 10.1385/MN:26:2-3:347. Mol Neurobiol. 2002. PMID: 12428764 Review.
-
Mutation causing severe myasthenia reveals functional asymmetry of AChR signature cystine loops in agonist binding and gating.J Clin Invest. 2003 Feb;111(4):497-505. doi: 10.1172/JCI16997. J Clin Invest. 2003. PMID: 12588888 Free PMC article.
-
Effect of Inhibiting p38 on HuR Involving in β-AChR Post-transcriptional Mechanisms in Denervated Skeletal Muscle.Cell Mol Neurobiol. 2019 Oct;39(7):1029-1037. doi: 10.1007/s10571-019-00698-0. Epub 2019 Jun 6. Cell Mol Neurobiol. 2019. PMID: 31172341 Free PMC article.
-
Current status of the congenital myasthenic syndromes.Neuromuscul Disord. 2012 Feb;22(2):99-111. doi: 10.1016/j.nmd.2011.10.009. Epub 2011 Nov 21. Neuromuscul Disord. 2012. PMID: 22104196 Free PMC article. Review.
-
HuR Mediates Changes in the Stability of AChR β-Subunit mRNAs after Skeletal Muscle Denervation.J Neurosci. 2015 Aug 5;35(31):10949-62. doi: 10.1523/JNEUROSCI.1043-15.2015. J Neurosci. 2015. PMID: 26245959 Free PMC article.
References
-
- Yu X-M, Hall ZW. Extracellular domains mediating ε subunit interactions of muscle acetylcholine receptor. Nature. 1991;352:64–67. - PubMed
-
- Verrall S, Hall ZW. The N-terminal domains of the acetylcholine receptor subunits contain recognition signals for the initial steps of receptor assembly. Cell. 1992;68:23–31. - PubMed
-
- Green WN, Wanamaker CP. The role of the cystine loop in acetylcholine receptor assembly. J Biol Chem. 1997;272:20945–20953. - PubMed
-
- Gu Y, Camacho P, Gardner P, Hall ZW. Identification of two amino acid residues in the epsilon subunit that promote mammalian muscle acetylcholine receptor assembly in COS cells. Neuron. 1991;6:879–887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

