A role for the Tec family tyrosine kinase Txk in T cell activation and thymocyte selection
- PMID: 10562318
- PMCID: PMC3207325
- DOI: 10.1084/jem.190.10.1427
A role for the Tec family tyrosine kinase Txk in T cell activation and thymocyte selection
Abstract
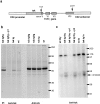
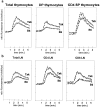
Recent data indicate that several members of the Tec family of protein tyrosine kinases function in antigen receptor signal transduction. Txk, a Tec family protein tyrosine kinase, is expressed in both immature and mature T cells and in mast cells. By overexpressing Txk in T cells throughout development, we found that Txk specifically augments the phospholipase C (PLC)-gamma1-mediated calcium signal transduction pathway upon T cell antigen receptor (TCR) engagement. Although Txk is structurally different from inducible T cell kinase (Itk), another Tec family member expressed in T cells, expression of the Txk transgene could partially rescue defects in positive selection and signaling in itk(-)(/)(-) mice. Conversely, in the itk(+/+) (wild-type) background, overexpression of Txk inhibited positive selection of TCR transgenic thymocytes, presumably due to induction of cell death. These results identify a role for Txk in TCR signal transduction, T cell development, and selection and suggest that the Tec family kinases Itk and Txk perform analogous functions.
Figures







Similar articles
-
Murine txk: a protein tyrosine kinase gene regulated by T cell activation.Oncogene. 1995 Jul 20;11(2):245-51. Oncogene. 1995. PMID: 7542761
-
Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk.Immunol Rev. 2009 Mar;228(1):93-114. doi: 10.1111/j.1600-065X.2008.00757.x. Immunol Rev. 2009. PMID: 19290923 Free PMC article. Review.
-
Transcriptional regulation of kinases downstream of the T cell receptor: another immunomodulatory mechanism of glucocorticoids.BMC Pharmacol Toxicol. 2014 Jul 3;15:35. doi: 10.1186/2050-6511-15-35. BMC Pharmacol Toxicol. 2014. PMID: 24993777 Free PMC article.
-
Reconstitution of Btk signaling by the atypical tec family tyrosine kinases Bmx and Txk.J Biol Chem. 1999 May 7;274(19):13577-85. doi: 10.1074/jbc.274.19.13577. J Biol Chem. 1999. PMID: 10224128
-
Tec family kinases Itk and Rlk / Txk in T lymphocytes: cross-regulation of cytokine production and T-cell fates.FEBS J. 2011 Jun;278(12):1980-9. doi: 10.1111/j.1742-4658.2011.08072.x. Epub 2011 Mar 29. FEBS J. 2011. PMID: 21362139 Free PMC article. Review.
Cited by
-
Bmx tyrosine kinase transgene induces skin hyperplasia, inflammatory angiogenesis, and accelerated wound healing.Mol Biol Cell. 2004 Sep;15(9):4226-33. doi: 10.1091/mbc.e04-03-0241. Epub 2004 Jun 30. Mol Biol Cell. 2004. PMID: 15229285 Free PMC article.
-
Differential expression of mRNA for Th1 and Th2 cytokine-associated transcription factors and suppressors of cytokine signalling in peripheral blood mononuclear cells of patients with atopic dermatitis.Clin Exp Immunol. 2004 Mar;135(3):505-10. doi: 10.1111/j.1365-2249.2004.02405.x. Clin Exp Immunol. 2004. PMID: 15008986 Free PMC article.
-
T-cell signaling regulated by the Tec family kinase, Itk.Cold Spring Harb Perspect Biol. 2010 Jul;2(7):a002287. doi: 10.1101/cshperspect.a002287. Epub 2010 Jun 2. Cold Spring Harb Perspect Biol. 2010. PMID: 20519342 Free PMC article. Review.
-
Topical Application of Temperature-Sensitive Gel Containing Caerin 1.1 and 1.9 Peptides on TC-1 Tumour-Bearing Mice Induced High-Level Immune Response in the Tumour Microenvironment.Front Oncol. 2021 Nov 11;11:754770. doi: 10.3389/fonc.2021.754770. eCollection 2021. Front Oncol. 2021. PMID: 34858827 Free PMC article.
-
Induction and stability of the anergic phenotype in T cells.Semin Immunol. 2013 Nov 15;25(4):313-20. doi: 10.1016/j.smim.2013.10.010. Epub 2013 Nov 5. Semin Immunol. 2013. PMID: 24211041 Free PMC article. Review.
References
-
- Sommers C.L., Huang K., Shores E.W., Grinberg A., Charlick D.A., Kozak C.A., Love P.E. Murine txka protein tyrosine kinase gene regulated by T cell activation. Oncogene. 1995;11:245–251. - PubMed
-
- Hu Q., Davidson D., Schwartzberg P.L., Macchiarini F., Lenardo M.J., Bluestone J., Matis L.A. Identification of Rlk, a novel protein tyrosine kinase with predominant expression in the T cell lineage. J. Biol. Chem. 1995;270:1928–1934. - PubMed
-
- Haire R.N., Litman G.W. The murine form of TXK, a novel TEC kinase expressed in thymus maps to chromosome 5. Mamm. Genome. 1995;6:476–480. - PubMed
-
- Tsukada S., Saffran D.C., Rawlings D.J., Parolini O., Allen R.C., Klisak I., Sparkes R.S., Kubagawa H., Mohandas T., Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. - PubMed
-
- Yamada N., Kawakami Y., Kimura H., Fukamachi H., Baier G., Altman A., Kato T., Inagaki Y., Kawakami T. Structure and expression of novel protein-tyrosine kinases, Emb and Emt, in hematopoietic cells. Biochem. Biophys. Res. Commun. 1993;192:231–240. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

