Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects
- PMID: 10562321
- PMCID: PMC2195693
- DOI: 10.1084/jem.190.10.1465
Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects
Abstract
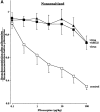
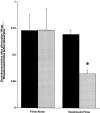
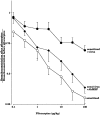
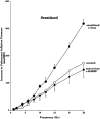
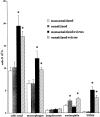
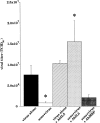
Asthma exacerbations, many of which are virus induced, are associated with airway eosinophilia. This may reflect altered inflammatory response to viruses in atopic individuals. Inhibitory M(2) muscarinic receptors (M(2)Rs) on the airway parasympathetic nerves limit acetylcholine release. Both viral infection and inhalational antigen challenge cause M(2)R dysfunction, leading to airway hyperresponsiveness. In antigen-challenged, but not virus-infected guinea pigs, M(2)R dysfunction is due to blockade of the receptors by the endogenous antagonist eosinophil major basic protein (MBP). We hypothesized that sensitization to a nonviral antigen before viral infection alters the inflammatory response to viral infection, so that M(2)R dysfunction and hyperreactivity are eosinophil mediated. Guinea pigs were sensitized to ovalbumin intraperitoneally, and 3 wk later were infected with parainfluenza. In sensitized, but not in nonsensitized animals, virus-induced hyperresponsiveness and M(2)R dysfunction were blocked by depletion of eosinophils with antibody to interleukin (IL)-5 or treatment with antibody to MBP. An additional and unexpected finding was that sensitization to ovalbumin caused a marked (80%) reduction in the viral content of the lungs. This was reversed by the antibody to IL-5, implicating a role for eosinophils in viral immunity.
Figures









References
-
- Empey D.W., Laitinen L.A., Jacobs L., Gold W.M., Nadel J.A. Mechanisms of bronchial hyperreactivity in normal subjects following upper respiratory tract infection. Am. Rev. Respir. Dis. 1976;113:523–527. - PubMed
-
- Nadel J.A., Barnes P.J. Autonomic regulation of the airways. Annu. Rev. Med. 1984;35:451–467. - PubMed
-
- Roffel A.F., Elzinga C.R., Zaagsma J. Muscarinic M3 receptors mediate contraction of human central and peripheral airway smooth muscle. Pulm. Pharmacol. 1990;3:47–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

