Teasing out the truth about collagen
- PMID: 10562329
- PMCID: PMC2269656
- DOI: 10.1111/j.1469-7793.1999.00001.x
Teasing out the truth about collagen
Abstract
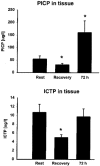
Of all of the non-mineral constituents of the mammalian body there is more collagen than anything else except water and possibly fat. Nevertheless our understanding of the physiology of collagen is rudimentary. All cells and tissues are supported by a network of collagen fibres, the arrangement of which appears to be specifically site adaptive. We know a lot about the biochemistry of collagen, and its many subtypes: for example, all collagen molecules are made within fibroblasts (or modifications of them such as osteocytes), then the oversized collagen molecule is secreted in a soluble form, with hydrophilic ends which are enzymatically cleaved to leave the insoluble core collagen (tropocollagen) beached in the extracellular space. We know that collagen is made relatively immortal by being cross-linked and rather impervious to proteolysis. However, we do not know much about what governs collagen synthesis or its breakdown in the human body. It is important to know, not simply because like Everest, collagen presents a large unignorable mass. We need to understand collagen metabolism in order to understand how we grow, adapt to the environment, maintain our adult shapes and then wrinkle and crumble as we age. Collagen diseases are relatively common and almost certainly if we knew more about how, for example, the collagen framework of bone is laid down and turned over we would understand much more about osteopenia of old age. The problem in finding out has been that collagen is so difficult to study. It turns over relatively slowly, and that part of it that is cross-linked and forms mature collagen is, it seems, with us for life come hell, high-water or famine. The body reduces to mainly skin and bone-collagen in extremis. Because the system as a whole is so sluggish, it is difficult to see changes in indices of collagen metabolism. However, not all the body collagen seems to be as fixed, and indeed collagen in some tissues must turn over, enabling remodelling and adaptation, rather quickly. Think about the stiffness and discomfort that accompanies un-accustomed exercise, which not only abates with time but ceases to occur once the exercise has become customary. What is happening to collagen protein turnover in these circumstances? One obvious way to study protein turnover, even of collagen, is to follow the incorporation of stable isotope markers such as proline into the tissue (although the breakdown is harder to quantify), but this is technically difficult and requires biopsy of the tissue in question. Another way is to follow the appearance in biological fluids of markers of collagen turnover. Since the propeptides which make collagen soluble are cleaved as collagen is deposited extracellularly, their concentration is an index of the rate of collagen synthesis; similarly when tropocollagen is degraded by extracellular proteases, specific N- and C-terminal fragments are released, the amount of which scales with the rate of collagen breakdown. These bits of collagen find their way into the blood. However, assaying them there introduces non-specificity and dilution, rendering interpretation difficult. The ideal would be to measure them in the extracellular fluid at the site of production. This of course is not easy in vivo. One of the delights of the paper by Langberg and colleagues in this issue of The Journal of Physiology (Langberg et al. 1999) is the sheer cheek with which the authors decided to use the microdialysis technique to do this. Microdialysis is a technique whereby a slowly perfused, thin-walled membranous tube is introduced into the extracellular space and the collected fluid assayed for molecules which have diffused into it. Until now the idea of using microdialysis to measure concentrations of molecules much bigger than 300 Da would be regarded as ludicrous. (ABSTRACT TRUNCATED)
Figures
Comment on
-
Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans.J Physiol. 1999 Nov 15;521 Pt 1(Pt 1):299-306. doi: 10.1111/j.1469-7793.1999.00299.x. J Physiol. 1999. PMID: 10562353 Free PMC article.
References
-
- Rennie MJ. In: Handbook of Physiology, Control of Energy Metabolism During Exerise. Terjung RL, editor. Bethesda, MD, USA: American Physiological Society; 1996. pp. 995–1035. chap. 22, section 12.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources