Basolateral proteinase-activated receptor (PAR-2) induces chloride secretion in M-1 mouse renal cortical collecting duct cells
- PMID: 10562330
- PMCID: PMC2269634
- DOI: 10.1111/j.1469-7793.1999.00003.x
Basolateral proteinase-activated receptor (PAR-2) induces chloride secretion in M-1 mouse renal cortical collecting duct cells
Abstract
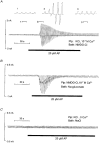
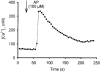
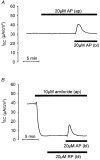
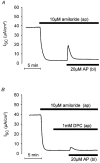
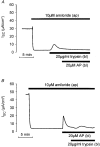
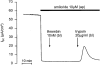
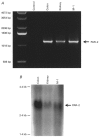
1. Using RT-PCR, Northern blot analysis, and immunocytochemistry, we confirmed renal expression of proteinase-activated receptor (PAR-2) and demonstrated its presence in native renal epithelial and in cultured M-1 mouse cortical collecting duct (CCD) cells. 2. We investigated the effects of a PAR-2 activating peptide (AP), corresponding to the tethered ligand that is exposed upon trypsin cleavage, and of trypsin on M-1 cells using patch-clamp, intracellular calcium (fura-2) and transepithelial short-circuit current (ISC) measurements. 3. In single M-1 cells, addition of AP elicited a concentration-dependent transient increase in the whole-cell conductance. Removal of extracellular Na+ had no effect while removal of Cl- prevented the stimulation of outward currents. The intracellular calcium concentration increased significantly upon application of AP while a Ca2+-free pipette solution completely abolished the electrical response to AP. 4. In confluent monolayers of M-1 cells, apical application of AP had no effect on ISC whereas subsequent basolateral application elicited a transient increase in ISC. This increase was not due to a stimulation of electrogenic Na+ absorption since the response was preserved in the presence of amiloride. 5. The ISC response to AP was reduced in the presence of the Cl- channel blocker diphenylamine-2-carboxylic acid on the apical side and abolished in the absence of extracellular Cl-. 6. Trypsin elicited similar responses to those to AP while application of a peptide (RP) with the reverse amino acid sequence of AP had no effect on whole-cell currents or ISC. 7. In conclusion, our data suggest that AP or trypsin stimulates Cl- secretion by Ca2+-activated Cl- channels in M-1 CCD cells by activating basolateral PAR-2.
Figures

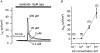


References
-
- Böhm SK, Khitin LM, Grady EF, Aponte G, Payan DG, Bunnett NW. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. Journal of Biological Chemistry. 1996a;271:22003–22016. - PubMed
-
- Chalfant ML, Peterson-Yantorno K, O'Brien TG, Civan MM. Regulation of epithelial Na+ channels from M-1 cortical collecting duct cells. American Journal of Physiology. 1996;271:F861–870. - PubMed
-
- Christine CW, Laskowski FH, Gitter AH, Beyenbach KW, Gross P, Frömter E. Anion channels in the apical membrane of collecting duct principal cells in culture. Cellular Physiology and Biochemistry. 1991;1:76–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

