The cytoplasmic chaperone hsp104 is required for conformational repair of heat-denatured proteins in the yeast endoplasmic reticulum
- PMID: 10564260
- PMCID: PMC25649
- DOI: 10.1091/mbc.10.11.3623
The cytoplasmic chaperone hsp104 is required for conformational repair of heat-denatured proteins in the yeast endoplasmic reticulum
Abstract
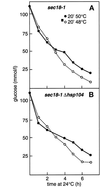
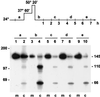
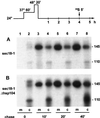
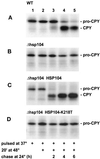
Severe heat stress causes protein denaturation in various cellular compartments. If Saccharomyces cerevisiae cells grown at 24 degrees C are preconditioned at 37 degrees C, proteins denatured by subsequent exposure to 48-50 degrees C can be renatured when the cells are allowed to recover at 24 degrees C. Conformational repair of vital proteins is essential for survival, because gene expression is transiently blocked after the thermal insult. Refolding of cytoplasmic proteins requires the Hsp104 chaperone, and refolding of lumenal endoplasmic reticulum (ER) proteins requires the Hsp70 homologue Lhs1p. We show here that conformational repair of heat-damaged glycoproteins in the ER of living yeast cells required functional Hsp104. A heterologous enzyme and a number of natural yeast proteins, previously translocated and folded in the ER and thereafter denatured by severe heat stress, failed to be refolded to active and secretion-competent structures in the absence of Hsp104 or when an ATP-binding site of Hsp104 was mutated. During recovery at 24 degrees C, the misfolded proteins persisted in the ER, although the secretory apparatus was fully functional. Hsp104 appears to control conformational repair of heat-damaged proteins even beyond the ER membrane.
Figures




Similar articles
-
Regulation and recovery of functions of Saccharomyces cerevisiae chaperone BiP/Kar2p after thermal insult.Eukaryot Cell. 2005 Dec;4(12):2008-16. doi: 10.1128/EC.4.12.2008-2016.2005. Eukaryot Cell. 2005. PMID: 16339719 Free PMC article.
-
The Hsp70 homologue Lhs1p is involved in a novel function of the yeast endoplasmic reticulum, refolding and stabilization of heat-denatured protein aggregates.J Cell Biol. 1997 May 19;137(4):813-24. doi: 10.1083/jcb.137.4.813. J Cell Biol. 1997. PMID: 9151684 Free PMC article.
-
Trehalose is required for conformational repair of heat-denatured proteins in the yeast endoplasmic reticulum but not for maintenance of membrane traffic functions after severe heat stress.Mol Microbiol. 2000 Jul;37(1):42-53. doi: 10.1046/j.1365-2958.2000.01970.x. Mol Microbiol. 2000. PMID: 10931304
-
Endoplasmic reticulum associated protein degradation: a chaperone assisted journey to hell.Biochim Biophys Acta. 2010 Jun;1803(6):694-705. doi: 10.1016/j.bbamcr.2010.02.005. Epub 2010 Feb 25. Biochim Biophys Acta. 2010. PMID: 20219571 Review.
-
Calnexin, calreticulin, and Bip/Kar2p in protein folding.Cold Spring Harb Symp Quant Biol. 1995;60:405-15. doi: 10.1101/sqb.1995.060.01.045. Cold Spring Harb Symp Quant Biol. 1995. PMID: 8824414 Review. No abstract available.
Cited by
-
Regulation and recovery of functions of Saccharomyces cerevisiae chaperone BiP/Kar2p after thermal insult.Eukaryot Cell. 2005 Dec;4(12):2008-16. doi: 10.1128/EC.4.12.2008-2016.2005. Eukaryot Cell. 2005. PMID: 16339719 Free PMC article.
-
Role of the HSP70 Co-Chaperone SIL1 in Health and Disease.Int J Mol Sci. 2021 Feb 4;22(4):1564. doi: 10.3390/ijms22041564. Int J Mol Sci. 2021. PMID: 33557244 Free PMC article. Review.
-
Rewiring of Signaling Networks Modulating Thermotolerance in the Human Pathogen Cryptococcus neoformans.Genetics. 2017 Jan;205(1):201-219. doi: 10.1534/genetics.116.190595. Epub 2016 Nov 18. Genetics. 2017. PMID: 27866167 Free PMC article.
-
From the baker to the bedside: yeast models of Parkinson's disease.Microb Cell. 2015 Jul 27;2(8):262-279. doi: 10.15698/mic2015.08.219. Microb Cell. 2015. PMID: 28357302 Free PMC article. Review.
-
Spiraling in Control: Structures and Mechanisms of the Hsp104 Disaggregase.Cold Spring Harb Perspect Biol. 2019 Aug 1;11(8):a034033. doi: 10.1101/cshperspect.a034033. Cold Spring Harb Perspect Biol. 2019. PMID: 30745294 Free PMC article. Review.
References
-
- Ammerer G. Expression of genes in yeast using the ADC1 promoter. Methods Enzymol. 1983;10:192–201. - PubMed
-
- Bonneaud M, Ozier-Kalogeropoulos O, Li G, Labouesse M, Minvielle-Sebastia L, Lacroute F. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast. 1991;7:609–615. - PubMed
-
- Caplan AJ, Cyr CM, Douglas MG. Ydj1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell. 1992;71:1143–1155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

