Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE
- PMID: 10565920
- PMCID: PMC85863
- DOI: 10.1128/JCM.37.12.3990-3996.1999
Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE
Abstract
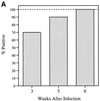
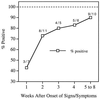
VlsE, the variable surface antigen of Borrelia burgdorferi, contains an immunodominant conserved region named IR(6). In the present study, the diagnostic performance of a peptide enzyme-linked immunosorbent assay (ELISA) based on a 26-mer synthetic peptide (C(6)) with the IR(6) sequence was explored. Sensitivity was assessed with serum samples (n = 210) collected from patients with clinically defined Lyme disease at the acute (early localized or early disseminated disease), convalescent, or late disease phase. The sensitivities for acute-, convalescent-, and late-phase specimens were 74% (29 of 39), 85 to 90% (34 of 40 to 35 of 39), and 100% (59 of 59), respectively. Serum specimens from early neuroborreliosis patients were 95% positive (19 of 20), and those from an additional group of patients with posttreatment Lyme disease syndrome yielded a sensitivity of 62% (8 of 13). To assess the specificity of the peptide ELISA, 77 serum samples from patients with other spirochetal or chronic infections, autoimmune diseases, or neurologic diseases and 99 serum specimens from hospitalized patients in an area where Lyme disease is not endemic were examined. Only two potential false positives from the hospitalized patients were found, and the overall specificity was 99% (174 of 176). Precision, which was assessed with a panel of positive and negative serum specimens arranged in blinded duplicates, was 100%. Four serum samples with very high anti-OspA antibody titers obtained from four monkeys given the OspA vaccine did not react with the C(6) peptide. This simple, sensitive, specific, and precise ELISA may contribute to alleviate some of the remaining problems in Lyme disease serodiagnosis. Because of its synthetic peptide base, it will be inexpensive to manufacture. It also will be applicable to serum specimens from OspA-vaccinated subjects.
Figures


References
-
- Barony G, Merrifield R B. The peptides: analysis, synthesis, & biology. New York, N.Y: Academic Press; 1980. pp. 3–285.
-
- Burkot T R, Schriefer M E, Larsen S A. Cross-reactivity to Borrelia burgdorferi proteins in serum samples from residents of a tropical country nonendemic for Lyme disease. J Infect Dis. 1997;175:466–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

