Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi
- PMID: 10565939
- PMCID: PMC85891
- DOI: 10.1128/JCM.37.12.4107-4112.1999
Production of monoclonal antibodies directed against the microsporidium Enterocytozoon bieneusi
Abstract
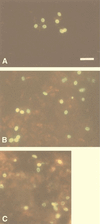
Several hybridomas producing antibodies detected by indirect immunofluorescence antibody test (IFAT) were established by fusion of mouse myeloma SP2/O with spleen cells from BALB/c mice immunized against whole spores (protocol 1) or chitinase-treated spores (protocol 2) of Enterocytozoon bieneusi and were cloned twice by limiting dilutions. Two monoclonal antibodies (MAbs), 3B82H2 from protocol 1, isotyped as immunoglobulin M (IgM), and 6E52D9 from protocol 2, isotyped as IgG, were expanded in both ascites and culture. IFAT with the MAbs showed that both MAbs reacted exclusively with the walls of the spores of E. bieneusi, strongly staining the surface of mature spores, and produced titers of greater than 4,096. Immunogold electron microscopy confirmed the specific reactivities of both antibodies. No cross-reaction, either with the spores of the other intestinal microsporidium species Encephalitozoon intestinalis or with yeast cells, bacteria, or any other intestinal parasites, was observed. The MAbs were used to identify E. bieneusi spores in fecal specimens from patients suspected of having intestinal microsporidiosis. The IFAT was validated against standard staining methods (Chromotrope 2R and Uvitex 2B) and PCR. We report here the first description and characterization of two MAbs specific for the spore wall of E. bieneusi. These MAbs have great potential for the demonstration and species determination of E. bieneusi, and their application in immunofluorescence identification of E. bieneusi in stool samples could offer a new diagnostic tool for clinical laboratories.
Figures




References
-
- Accoceberry I, Carrière J, Thellier M, Biligui S, Danis M, Datry A. Rat model for the human intestinal microsporidian Enterocytozoon bieneusi. J Eukaryot Microbiol. 1997;44:83S. - PubMed
-
- Accoceberry I, Greiner P, Thellier M, Achbarou A, Biligui S, Danis M, Datry A. Rabbit model for human intestinal microsporidia. J Eukaryot Microbiol. 1997;44:82S. - PubMed
-
- Asmuth D M, DeGirolami P C, Federman M, Ezratty C R, Pleskow D K, Desai G, Wanke C A. Clinical features of microsporidiosis in patients with AIDS. Clin Infect Dis. 1994;18:819–825. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

