PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner
- PMID: 10567539
- PMCID: PMC84898
- DOI: 10.1128/MCB.19.12.8136
PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner
Erratum in
- Mol Cell Biol 2000 Mar;20(5):1897
Abstract
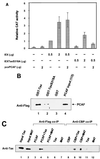
Recent studies have shown that the p300/CREB binding protein (CBP)-associated factor (PCAF) is involved in transcriptional activation. PCAF activity has been shown strongly associated with histone acetyltransferase (HAT) activity. In this report, we present evidence for a HAT-independent transcription function that is activated in the presence of the human T-cell leukemia virus type 1 (HTLV-1) Tax protein. In vitro and in vivo GST-Tax pull-down and coimmunoprecipitation experiments demonstrate that there is a direct interaction between Tax and PCAF, independent of p300/CBP. PCAF can be recruited to the HTLV-1 Tax responsive element in the presence of Tax, and PCAF cooperates with Tax in vivo to activate transcription from the HTLV-1 LTR over 10-fold. Point mutations at Tax amino acid 318 (TaxS318A) or 319 to 320 (Tax M47), which have decreased or no activity on the HTLV-1 promoter, are defective for PCAF binding. Strikingly, the ability of PCAF to stimulate Tax transactivation is not solely dependent on the PCAF HAT domain. Two independent PCAF HAT mutants, which knock out acetyltransferase enzyme activity, activate Tax transactivation to approximately the same level as wild-type PCAF. In contrast, p300 stimulation of Tax transactivation is HAT dependent. These studies provide experimental evidence that PCAF contains a coactivator transcription function independent of the HAT activity on the viral long terminal repeat.
Figures






References
-
- Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. - PubMed
-
- Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
-
- Bannister A J, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
