Pbx-Hox heterodimers recruit coactivator-corepressor complexes in an isoform-specific manner
- PMID: 10567547
- PMCID: PMC84906
- DOI: 10.1128/MCB.19.12.8219
Pbx-Hox heterodimers recruit coactivator-corepressor complexes in an isoform-specific manner
Abstract
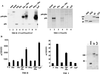
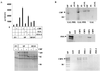
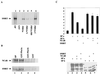
Homeobox (hox) proteins have been shown to regulate cell fate and segment identity by promoting the expression of specific genetic programs. In contrast to their restricted biological action in vivo, however, most homeodomain factors exhibit promiscuous DNA binding properties in vitro, suggesting a requirement for additional cofactors that enhance target site selectivity. In this regard, the pbx family of homeobox genes has been found to heterodimerize with and thereby augment the DNA binding activity of certain hox proteins on a subset of potential target sites. Here we examine the transcriptional properties of a forced hox-pbx heterodimer containing the pancreas-specific orphan homeobox factor pdx fused to pbx-1a. Compared to the pdx monomer, the forced pdx-pbx1a dimer, displayed 10- to 20-fold-higher affinity for a consensus hox-pbx binding site but was completely unable to bind a hox monomer recognition site. The pdx-pbx dimer stimulated target gene expression via an N-terminal trans-activation domain in pdx that interacts with the coactivator CREB binding protein. The pdx-pbx dimer was also found to repress transcription via a C-terminal domain in pbx-1a that associates with the corepressors SMRT and NCoR. The transcriptional properties of the pdx-pbx1 complex appear to be regulated at the level of alternative splicing; a pdx-pbx polypeptide containing the pbx1b isoform, which lacks the C-terminal extension in pbx1a, was unable to repress target gene expression via NCoR-SMRT. Since pbx1a and pbx1b are differentially expressed in endocrine versus exocrine compartments of the adult pancreas, our results illustrate a novel mechanism by which pbx proteins may modulate the expression of specific genetic programs, either positively or negatively, during development.
Figures
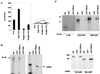

References
-
- Alland L, Muhle R, Hou H, Potes J, Chin L, Schreiber-Agus N, DePinho R. A Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. - PubMed
-
- Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
-
- Chan S, Jaffe L, Capovilla M, Botas J, Mann R. The DNA binding specificity of ultrabithorax is modulated by cooperative interactions with extradenticle, another homeoprotein. Cell. 1994;78:603–615. - PubMed
-
- Chang C, Shen W, Rozenfeld S, Lawrence H, Largman C, Cleary M. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 1995;9:663–674. - PubMed
-
- Chen J, Evans R. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
