Characterization of a novel member of the DOK family that binds and modulates Abl signaling
- PMID: 10567556
- PMCID: PMC84915
- DOI: 10.1128/MCB.19.12.8314
Characterization of a novel member of the DOK family that binds and modulates Abl signaling
Abstract
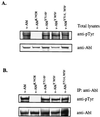
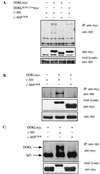
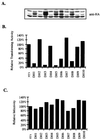
A novel member of the p62(dok) family of proteins, termed DOKL, is described. DOKL contains features of intracellular signaling molecules, including an N-terminal PH (pleckstrin homology) domain, a central PTB (phosphotyrosine binding) domain, and a C-terminal domain with multiple potential tyrosine phosphorylation sites and proline-rich regions, which might serve as docking sites for SH2- and SH3-containing proteins. The DOKL gene is predominantly expressed in bone marrow, spleen, and lung, although low-level expression of the RNA can also be detected in other tissues. DOKL and p62(dok) bind through their PTB domains to the Abelson tyrosine kinase in a kinase-dependent manner in both yeast and mammalian cells. DOKL is phosphorylated by the Abl tyrosine kinase in vivo. In contrast to p62(dok), DOKL lacks YxxP motifs in the C terminus and does not bind to Ras GTPase-activating protein (RasGAP) upon phosphorylation. Overexpression of DOKL, but not p62(dok), suppresses v-Abl-induced mitogen-activated protein (MAP) kinase activation but has no effect on constitutively activated Ras- and epidermal growth factor-induced MAP kinase activation. The inhibitory effect requires the PTB domain of DOKL. Finally, overexpression of DOKL in NIH 3T3 cells inhibits the transforming activity of v-Abl. These results suggest that DOKL may modulate Abl function.
Figures








References
-
- Alexandropoulos K, Baltimore D. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 1996;10:1341–1355. - PubMed
-
- Bhat A, Johnson K J, Oda T, Corbin A S, Druker B J. Interactions of p62(dok) with p210(bcr-abl) and bcr-abl-associated proteins. J Biol Chem. 1998;273:32360–32368. - PubMed
-
- Bhat A, Kolibaba K, Oda T, Ohno-Jones S, Heaney C, Druker B J. Interactions of CBL with BCR-ABL and CRKL in BCR-ABL-transformed myeloid cells. J Biol Chem. 1997;272:16170–16175. - PubMed
-
- Carpino N, Wisniewski D, Strife A, Marshak D, Kobayashi R, Stillman B, Clarkson B. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88:197–204. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
