Role of endogenous interleukin-18 in resolving wild-type and attenuated Salmonella typhimurium infections
- PMID: 10569733
- PMCID: PMC97025
- DOI: 10.1128/IAI.67.12.6242-6248.1999
Role of endogenous interleukin-18 in resolving wild-type and attenuated Salmonella typhimurium infections
Abstract
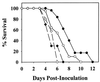
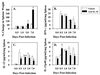
The stimulation of gamma interferon (IFN-gamma) has been shown to be essential in resolving infections by intracellular pathogens. As such, several different cytokines including, interleukin-12 (IL-12) and IL-18, can induce IFN-gamma. To resolve Salmonella infections, the stimulation of IL-12 and IFN-gamma are important for mediating its clearance. In this present study, the relevance of IL-18 in protection against oral challenge with Salmonella typhimurium was investigated to determine the role of this IFN-gamma-promoting cytokine. Rabbit anti-murine IL-18 antisera was generated and administered prior to the oral challenge of BALB/c and IL-12p40-deficient knockout (IL-12KO) mice with a wild-type S. typhimurium strain. The median survival time was reduced by 2 days for the anti-IL-18-treated BALB/c mice, while no significant reduction in survival rate for the anti-IL-18-treated IL-12KO mice was observed compared to vehicle-treated mice. To investigate the contribution of IL-18 to resolving Salmonella infections, an attenuated aro-negative mutant (H647) was orally administered to BALB/c mice. This Salmonella infection induced both IL-12 and IFN-gamma in both the Peyer's patches and the spleens. In vehicle-treated mice, Peyer's patch IL-12 peaked by 24 h, while IL-18 levels peaked at 3 days, suggesting sequential support by these cytokines for IFN-gamma. Anti-IL-18 treatment exerted its greatest effect upon the mucosal compartment, limiting early IFN-gamma production. However, anti-IL-18 treatment had little effect upon splenic IFN-gamma levels until late in the response. Infection of IL-12KO mice with H647 strain induced IFN-gamma, but it was not supported by IL-18, although IL-18 levels were reduced by this treatment. These results suggest that IL-18 does contribute to the clearance of S. typhimurium and that endogenously induced IL-18 could not substitute for IL-12.
Figures


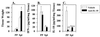
References
-
- Ahn H-J, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, Clark S, Kurimoto M, Okamura H, Fujiwara H. A mechanism underlying synergy between IL-12 and IFN-γ-inducing factor in enhanced production of IFN-γ. J Immunol. 1997;159:2125–2131. - PubMed
-
- Bohn E, Sing A, Zumbihl R, Bielfeldt C, Okamura H, Kurimoto M, Heesemann J, Autenrieth I B. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of bacterial infection in mice. J Immunol. 1998;160:299–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

