Mutants of Escherichia coli heat-labile toxin act as effective mucosal adjuvants for nasal delivery of an acellular pertussis vaccine: differential effects of the nontoxic AB complex and enzyme activity on Th1 and Th2 cells
- PMID: 10569737
- PMCID: PMC97029
- DOI: 10.1128/IAI.67.12.6270-6280.1999
Mutants of Escherichia coli heat-labile toxin act as effective mucosal adjuvants for nasal delivery of an acellular pertussis vaccine: differential effects of the nontoxic AB complex and enzyme activity on Th1 and Th2 cells
Abstract
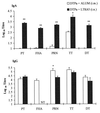
Mucosal delivery of vaccines is dependent on the identification of safe and effective adjuvants that can enhance the immunogenicity of protein antigens administered by nasal or oral routes. In this study we demonstrate that two mutants of Escherichia coli heat-labile toxin (LT), LTK63, which lacks ADP-ribosylating activity, and LTR72, which has partial enzyme activity, act as potent mucosal adjuvants for the nasal delivery of an acellular pertussis (Pa) vaccine. Both LTK63 and LTR72 enhanced antigen-specific serum immunoglobulin G (IgG), secretory IgA, and local and systemic T-cell responses. Furthermore, using the murine respiratory challenge model for infection with Bordetella pertussis, we demonstrated that a nasally delivered diphtheria, tetanus, and acellular pertussis (DTPa) combination vaccine formulated with LTK63 as an adjuvant conferred a high level of protection, equivalent to that generated with a parenterally delivered DTPa vaccine formulated with alum. This study also provides significant new information on the roles of the binding and enzyme components of LT in the modulation of Th1 and Th2 responses. LTK63, which lacks enzyme activity, promoted T-cell responses with a mixed Th1-Th2 profile, but LTR72, which retains partial enzyme activity, and the wild-type toxin, especially at low dose, induced a more polarized Th2-type response and very high IgA and IgG antibody titers. Our findings suggest that the nontoxic AB complex has broad adjuvant activity for T-cell responses and that the ADP-ribosyltransferase activity of the A subunit also appears to modulate cytokine production, but its effect on T-cell subtypes, as well as enhancing, may be selectively suppressive.
Figures



Similar articles
-
Modulation of innate and acquired immune responses by Escherichia coli heat-labile toxin: distinct pro- and anti-inflammatory effects of the nontoxic AB complex and the enzyme activity.J Immunol. 2000 Nov 15;165(10):5750-9. doi: 10.4049/jimmunol.165.10.5750. J Immunol. 2000. PMID: 11067933
-
Alternative diphtheria, tetanus and whooping cough immunization schedule to evoke a Th2 tetanus and a Th1 pertussis immune response.Microbes Infect. 2004 Apr;6(5):481-4. doi: 10.1016/j.micinf.2004.01.004. Microbes Infect. 2004. PMID: 15109963
-
Intranasal immunization with outer membrane vesicle pertussis vaccine confers broad protection through mucosal IgA and Th17 responses.Sci Rep. 2020 Apr 30;10(1):7396. doi: 10.1038/s41598-020-63998-2. Sci Rep. 2020. PMID: 32355188 Free PMC article.
-
Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants.Vaccine. 2001 Mar 21;19(17-19):2534-41. doi: 10.1016/s0264-410x(00)00553-3. Vaccine. 2001. PMID: 11257389 Review.
-
Reduced-antigen, combined diphtheria, tetanus and acellular pertussis vaccine, adsorbed (Boostrix®): a review of its properties and use as a single-dose booster immunization.Drugs. 2012 Sep 10;72(13):1765-91. doi: 10.2165/11209630-000000000-00000. Drugs. 2012. PMID: 22931522 Review.
Cited by
-
Transcutaneous immunization with a Vibrio cholerae O1 Ogawa synthetic hexasaccharide conjugate following oral whole-cell cholera vaccination boosts vibriocidal responses and induces protective immunity in mice.Clin Vaccine Immunol. 2012 Apr;19(4):594-602. doi: 10.1128/CVI.05689-11. Epub 2012 Feb 22. Clin Vaccine Immunol. 2012. PMID: 22357651 Free PMC article.
-
The LTR72 mutant of heat-labile enterotoxin of Escherichia coli enhances the ability of peptide antigens to elicit CD4(+) T cells and secrete gamma interferon after coapplication onto bare skin.Infect Immun. 2002 Jun;70(6):3012-9. doi: 10.1128/IAI.70.6.3012-3019.2002. Infect Immun. 2002. PMID: 12010992 Free PMC article.
-
Role of T cells in the adjuvant effect of Bacillus firmus on the immune system of mice: intranasal and intratracheal immunization study with ovalbumin.Folia Microbiol (Praha). 2003;48(3):427-34. doi: 10.1007/BF02931379. Folia Microbiol (Praha). 2003. PMID: 12879759
-
Mucosal immunization with a genetically engineered pertussis toxin S1 fragment-cholera toxin subunit B chimeric protein.Infect Immun. 2003 Apr;71(4):2272-5. doi: 10.1128/IAI.71.4.2272-2275.2003. Infect Immun. 2003. PMID: 12654855 Free PMC article.
-
Lipid-Based Particles: Versatile Delivery Systems for Mucosal Vaccination against Infection.Front Immunol. 2018 Mar 7;9:431. doi: 10.3389/fimmu.2018.00431. eCollection 2018. Front Immunol. 2018. PMID: 29563912 Free PMC article. Review.
References
-
- Almeida A J, Alpar H O. Nasal delivery of vaccines. J Drug Targeting. 1996;3:455–467. - PubMed
-
- Cahill E S, O'Hagan D T, Illum L, Barnard A, Mills K H G, Redhead K. Immune responses and protection against Bordetella pertussisinfection after intranasal immunization of mice with filamentous haemagglutinin in solution or incorporated in biodegradable microparticles. Vaccine. 1995;13:455–462. - PubMed
-
- Cahill E S, O'Hagan D T, Illum L, Redhead K. Mice are protected against Bordetella pertussisinfection by intra-nasal immunization with filamentous haemagglutinin. FEMS Microbiol Lett. 1993;107:211–216. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

