Selective recruitment of T-cell subsets to the udder during staphylococcal and streptococcal mastitis: analysis of lymphocyte subsets and adhesion molecule expression
- PMID: 10569740
- PMCID: PMC97032
- DOI: 10.1128/IAI.67.12.6293-6302.1999
Selective recruitment of T-cell subsets to the udder during staphylococcal and streptococcal mastitis: analysis of lymphocyte subsets and adhesion molecule expression
Abstract
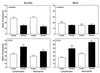
During bacterial infection of the bovine mammary gland, large numbers of leukocytes migrate into the udder, resulting in the establishment of a host response against the pathogen. Currently, the specific leukocyte populations mediating this immune response are not well defined. In the studies described here, we analyzed blood and milk from healthy cows and cows with naturally occurring mastitis to determine if distinct alphabeta and gammadelta T-lymphocyte subsets were involved in the response of the udder to a mastitis pathogen and if the type of mastitis pathogen influenced the subset composition of these responding leukocytes. Although blood samples from cows with confirmed staphylococcal and streptococcal mastitis were characterized by increased numbers of gammadelta T cells, the most dramatic changes in leukocyte distributions occurred in milk samples from these cows, with a 75% increase in alphabeta T-cell levels and a 100% increase in gammadelta T-cell levels relative to the levels in milk samples from healthy animals. Interestingly, the increase in alphabeta T-cell numbers observed in milk from cows with staphylococcal mastitis was primarily due to increased numbers of CD4(+) T cells, while the increase in alphabeta T-cell numbers observed in cows with streptococcal mastitis was due to a parallel increase in both CD4(+) and CD8(+) T-cell numbers. The increased numbers of gammadelta T cells in milk from cows with staphylococcal and streptococcal mastitis were due to a selective recruitment of a distinct gammadelta T-cell subset (GD3.1(+)), while no change in the numbers of GD197(+) gammadelta T cells was observed. We also analyzed adhesion protein expression on blood and milk leukocytes and found that, in comparison to the situation for healthy cows, L-selectin was down-regulated and CD18 was up-regulated on leukocytes from cows with mastitis. Thus, shedding of L-selectin and up-regulation of CD18 by neutrophils may provide a sensitive indicator of early inflammatory responses during bovine mastitis. Overall, these studies suggest that distinct alphabeta and gammadelta T-cell subsets are involved in the host defense of the udder against mastitis infection and that selective recruitment of these T-cell subsets depends on the infectious agent involved.
Figures




References
-
- Ali H, Haribabu B, Richardson R M, Snyderman R. Mechanisms of inflammation and leukocyte activation. Med Clin N Am. 1997;81:1–28. - PubMed
-
- Boismenu R, Havran W L. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–1255. - PubMed
-
- Boismenu R, Havran W L. An innate view of gamma delta T cells. Curr Opin Immunol. 1997;9:57–63. - PubMed
-
- Borregaard N, Kjeldsen L, Sengelov H, Diamond M S, Springer T A, Anderson H C, Kishimoto T K, Bainton D F. Changes in subcellular localization and surface expression of L-selectin, alkaline phosphatase, and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J Leukoc Biol. 1994;56:80–87. - PubMed
-
- Chien Y H, Jores R, Crowley M P. Recognition by gamma/delta T cells. Annu Rev Immunol. 1996;14:511–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

