Group B streptococcal surface proteins as targets for protective antibodies: identification of two novel proteins in strains of serotype V
- PMID: 10569749
- PMCID: PMC97041
- DOI: 10.1128/IAI.67.12.6350-6357.1999
Group B streptococcal surface proteins as targets for protective antibodies: identification of two novel proteins in strains of serotype V
Abstract
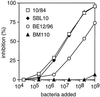
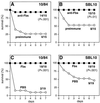
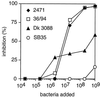
Strains of group B streptococcus (GBS) express surface proteins that confer protective immunity. In particular, most strains of the four classical capsular serotypes (Ia, Ib, II, and III) express either of the Rib and alpha proteins, two members of the same protein family. Here, we report a study of surface proteins expressed by strains of serotype V, which has recently emerged as an important serotype among GBS strains causing serious disease. Two novel GBS proteins were identified, purified, and characterized. One of these proteins, designated Fbs, was immunologically unrelated to other GBS surface proteins. This approximately 110-kDa protein was found in 15 of 49 (31%) type V isolates but in few strains of other serotypes. The Fbs proteins expressed by different strains showed limited variation in size. The most common surface protein among type V strains, found in 29 of 49 (59%) isolates, was designated Rib-like, since it cross-reacted with Rib but was not immunologically identical to Rib. Characterization of this Rib-like protein showed that the N-terminal sequence (12 residues) was identical to that of alpha, although these two proteins lacked cross-reactivity. The biochemical and immunological properties of the Rib-like GBS protein indicate that it is closely related to the R28 protein of Streptococcus pyogenes. Importantly, passive and active immunization experiments with mice showed that the Fbs and Rib-like proteins are targets for protective antibodies. These two proteins are therefore of interest for analysis of pathogenic mechanisms and for vaccine development.
Figures

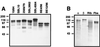

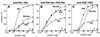
References
-
- Baker C J, Edwards M S. Group B streptococcal infection. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and the newborn infant. W. B. Philadelphia, Pa: Saunders Company; 1995. pp. 980–1054.
-
- Bevanger L, Naess A I. Mouse-protective antibodies against the Ibc proteins of group B streptococci. Acta Pathol Microbiol Immunol Scand Sect B. 1985;93:121–124. - PubMed
-
- Blumberg H M, Stephens D S, Modansky M, Erwin M, Elliot J, Facklam R R, Schuchat A, Baughman W, Farley M M. Invasive group B streptococcal disease: the emergence of serotype V. J Infect Dis. 1996;173:365–373. - PubMed
-
- Domingo P, Barquet N, Alvarez M, Coll P, Nava J, Garau J. Group B streptococcal meningitis in adults: report of twelve cases and review. Clin Infect Dis. 1997;25:1180–1187. - PubMed
-
- Edwards M S, Kasper D L, Jennings H J, Baker C J, Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982;128:1278–1283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

