Role of nitric oxide in the enhancement of pentylenetetrazole-induced seizures caused by Shigella dysenteriae
- PMID: 10569751
- PMCID: PMC97043
- DOI: 10.1128/IAI.67.12.6364-6368.1999
Role of nitric oxide in the enhancement of pentylenetetrazole-induced seizures caused by Shigella dysenteriae
Abstract
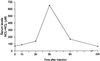
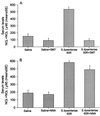
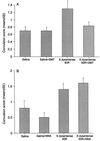
Convulsions and encephalopathy are frequent complications of childhood shigellosis. We studied the role of nitric oxide (NO) in Shigella-related seizures in an animal model. Pretreatment of mice with Shigella dysenteriae 60R sonicate elevated serum NO levels and enhanced the convulsive response to pentylenetetrazole (PTZ), as indicated by a higher mean convulsion score and a higher number of mice responding with seizures. Treatment of the mice with S-methylisothiourea sulfate (SMT), a potent inhibitor of inducible NO synthase (NOS), prevented the elevation of serum NO levels and concomitantly reduced the enhanced response to PTZ. The mean convulsion scores were 0.7, 0.7, 1.3, and 0.8 for mice treated with saline, saline and SMT, S. dysenteriae 60R sonicate, and S. dysenteriae 60R sonicate with SMT, respectively (P = 0.001 for 60R sonicate versus saline and P = 0.013 for 60R sonicate versus 60R sonicate with SMT). The corresponding seizure rates were 40, 44, 75, and 47% for saline, saline with SMT, S. dysenteriae 60R sonicate, and S. dysenteriae 60R sonicate with SMT, respectively (P = 0.0004 for 60R sonicate versus saline and P = 0.005 for 60R sonicate versus 60R sonicate with SMT). In contrast, injection of N-nitro-L-arginine, a selective inhibitor of constitutive NOS, neither abolished the elevation of serum NO nor attenuated the enhancement of seizures. These findings indicate that NO, induced by S. dysenteriae 60R sonicate, is involved in enhancing the susceptibility to seizures caused by S. dysenteriae.
Figures
References
-
- Ashkenazi S, Bellah G, Cleary T G. Hallucinations as an initial manifestation of childhood shigellosis. J Pediatr. 1989;114:95–96. - PubMed
-
- Ashkenazi S, Dinari G, Zevulunov A, Nitzan M. Convulsions in childhood shigellosis. Clinical and laboratory features in 153 children. Am J Dis Child. 1987;141:208–210. - PubMed
-
- Barrett T J, Potter M E, Strockline N E. Evidence for participation of the macrophage in Shiga-like toxin II-induced lethality in mice. Microb Pathog. 1990;9:95–103. - PubMed
-
- Barrett-Connor E, Connor J D. Extraintestinal manifestations of shigellosis. Am J Gastroenterol. 1970;53:234–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

