Regulation of human CD4(+) alphabeta T-cell-receptor-positive (TCR(+)) and gammadelta TCR(+) T-cell responses to Mycobacterium tuberculosis by interleukin-10 and transforming growth factor beta
- PMID: 10569764
- PMCID: PMC97056
- DOI: 10.1128/IAI.67.12.6461-6472.1999
Regulation of human CD4(+) alphabeta T-cell-receptor-positive (TCR(+)) and gammadelta TCR(+) T-cell responses to Mycobacterium tuberculosis by interleukin-10 and transforming growth factor beta
Abstract
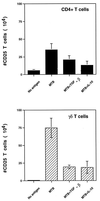
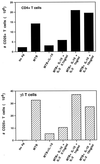
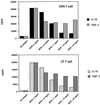
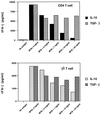
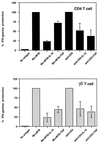
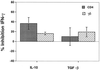
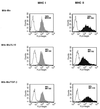
Mycobacterium tuberculosis is the etiologic agent of human tuberculosis and is estimated to infect one-third of the world's population. Control of M. tuberculosis requires T cells and macrophages. T-cell function is modulated by the cytokine environment, which in mycobacterial infection is a balance of proinflammatory (interleukin-1 [IL-1], IL-6, IL-8, IL-12, and tumor necrosis factor alpha) and inhibitory (IL-10 and transforming growth factor beta [TGF-beta]) cytokines. IL-10 and TGF-beta are produced by M. tuberculosis-infected macrophages. The effect of IL-10 and TGF-beta on M. tuberculosis-reactive human CD4(+) and gammadelta T cells, the two major human T-cell subsets activated by M. tuberculosis, was investigated. Both IL-10 and TGF-beta inhibited proliferation and gamma interferon production by CD4(+) and gammadelta T cells. IL-10 was a more potent inhibitor than TGF-beta for both T-cell subsets. Combinations of IL-10 and TGF-beta did not result in additive or synergistic inhibition. IL-10 inhibited gammadelta and CD4(+) T cells directly and inhibited monocyte antigen-presenting cell (APC) function for CD4(+) T cells and, to a lesser extent, for gammadelta T cells. TGF-beta inhibited both CD4(+) and gammadelta T cells directly and had little effect on APC function for gammadelta and CD4(+) T cells. IL-10 down-regulated major histocompatibility complex (MHC) class I, MHC class II, CD40, B7-1, and B7-2 expression on M. tuberculosis-infected monocytes to a greater extent than TGF-beta. Neither cytokine affected the uptake of M. tuberculosis by monocytes. Thus, IL-10 and TGF-beta both inhibited CD4(+) and gammadelta T cells but differed in the mechanism used to inhibit T-cell responses to M. tuberculosis.
Figures

References
-
- Ahuja S S, Paliogianni F, Yamada H, Balow J E, Boumpas D T. Effect of transforming growth factor-beta on early and late activation events in human T cells. J Immunol. 1993;150:3109–3118. - PubMed
-
- Balaji K N, Schwander S, Rich E A, Boom W H. Alveolar macrophages as accessory cells for human γδ T cells activated by M. tuberculosis. J Immunol. 1995;154:5959–5968. - PubMed
-
- Barnes P F, Mistry S D, Cooper C L, Pirmez C, Rea T H, Modlin R L. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989;142:1114–1119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

