Microtubules are associated with intracellular movement and spread of the periodontopathogen Actinobacillus actinomycetemcomitans
- PMID: 10569770
- PMCID: PMC97062
- DOI: 10.1128/IAI.67.12.6518-6525.1999
Microtubules are associated with intracellular movement and spread of the periodontopathogen Actinobacillus actinomycetemcomitans
Abstract
Actinobacillus actinomycetemcomitans SUNY 465, the invasion prototype strain, enters epithelial cells by an actin-dependent mechanism, escapes from the host cell vacuole, and spreads intracellularly and to adjacent epithelial cells via intercellular protrusions. Internalized organisms also egress from host cells into the assay medium via protrusions that are associated with just a single epithelial cell. Here we demonstrate that agents which inhibit microtubule polymerization (e.g., colchicine) and those which stabilize polymerized microtubules (e.g., taxol) both increase markedly the number of intracellular A. actinomycetemcomitans organisms. Furthermore, both colchicine and taxol prevented the egression of A. actinomycetemcomitans from host cells into the assay medium. Immunofluorescence microscopy revealed that protrusions that mediate the bacterial spread contain microtubules. A. actinomycetemcomitans SUNY 465 and 652, strains that are both invasive and egressive, interacted specifically with the plus ends (growing ends) of the filaments of microtubule asters in a KB cell extract. By contrast, neither A. actinomycetemcomitans 523, a strain that is invasive but not egressive, nor Haemophilus aphrophilus, a noninvasive oral bacterium with characteristics similar to those of A. actinomycetemcomitans, bound to microtubules. Together these data suggest that microtubules function in the spread and movement of A. actinomycetemcomitans and provide the first evidence that host cell dispersion of an invasive bacterium may involve the usurption of host cell microtubules.
Figures

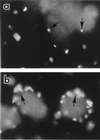

 ). Bacteria do not accumulate in
the assay medium if either taxol (□) or colchicine (■) is present.
Bars represent additive values (i.e., the 60-min bar represents the
40 × 103 CFU recovered at 30 min plus 20 ×
103 CFU recovered during the next 30 min, and so on) from a
typical experiment carried out in quadruplicate. The standard deviation
was less than 20% of the mean in all cases. The inset shows SUNY 465
in untreated and taxol-treated cells at time zero (□) and 180 min
(
). Bacteria do not accumulate in
the assay medium if either taxol (□) or colchicine (■) is present.
Bars represent additive values (i.e., the 60-min bar represents the
40 × 103 CFU recovered at 30 min plus 20 ×
103 CFU recovered during the next 30 min, and so on) from a
typical experiment carried out in quadruplicate. The standard deviation
was less than 20% of the mean in all cases. The inset shows SUNY 465
in untreated and taxol-treated cells at time zero (□) and 180 min
( ). (b) SUNY 465 recovered from KB cells after treatment with
cytochalasin D or brefeldin A. ■, control cells;
). (b) SUNY 465 recovered from KB cells after treatment with
cytochalasin D or brefeldin A. ■, control cells;  , treated
cells. KB cells were treated with brefeldin A prior to infection,
whereas cytochalasin D was added 90 min after infection.
, treated
cells. KB cells were treated with brefeldin A prior to infection,
whereas cytochalasin D was added 90 min after infection.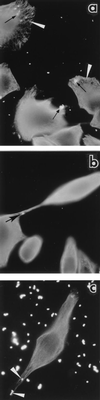

Similar articles
-
Actinobacillus actinomycetemcomitans may utilize either actin-dependent or actin-independent mechanisms of invasion.Oral Microbiol Immunol. 1999 Jun;14(3):137-42. doi: 10.1034/j.1399-302x.1999.140301.x. Oral Microbiol Immunol. 1999. PMID: 10495707
-
Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process.Infect Immun. 1996 Aug;64(8):2988-97. doi: 10.1128/iai.64.8.2988-2997.1996. Infect Immun. 1996. PMID: 8757825 Free PMC article.
-
Requirements for invasion of epithelial cells by Actinobacillus actinomycetemcomitans.Infect Immun. 1993 Apr;61(4):1239-45. doi: 10.1128/iai.61.4.1239-1245.1993. Infect Immun. 1993. PMID: 8454326 Free PMC article.
-
Models of invasion of enteric and periodontal pathogens into epithelial cells: a comparative analysis.Crit Rev Oral Biol Med. 1997;8(4):389-409. doi: 10.1177/10454411970080040301. Crit Rev Oral Biol Med. 1997. PMID: 9391752 Review.
-
Characteristics of Actinobacillus actinomycetemcomitans invasion of and adhesion to cultured epithelial cells.Adv Dent Res. 1995 Feb;9(1):55-62. doi: 10.1177/08959374950090011001. Adv Dent Res. 1995. PMID: 7669215 Review.
Cited by
-
Beyond good and evil in the oral cavity: insights into host-microbe relationships derived from transcriptional profiling of gingival cells.J Dent Res. 2008 Mar;87(3):203-23. doi: 10.1177/154405910808700302. J Dent Res. 2008. PMID: 18296603 Free PMC article. Review.
-
Shigella deliver an effector protein to trigger host microtubule destabilization, which promotes Rac1 activity and efficient bacterial internalization.EMBO J. 2002 Jun 17;21(12):2923-35. doi: 10.1093/emboj/cdf319. EMBO J. 2002. PMID: 12065406 Free PMC article.
-
Nocodazole inhibits macronuclear infection with Holospora obtusa in Paramecium caudatum.Protoplasma. 2005 Dec;226(3-4):147-53. doi: 10.1007/s00709-005-0121-7. Epub 2005 Dec 12. Protoplasma. 2005. PMID: 16333573
-
Orientia tsutsugamushi: A neglected but fascinating obligate intracellular bacterial pathogen.PLoS Pathog. 2017 Dec 7;13(12):e1006657. doi: 10.1371/journal.ppat.1006657. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29216334 Free PMC article. Review. No abstract available.
-
The intracellular bacterium Orientia tsutsugamushi uses the autotransporter ScaC to activate BICD adaptors for dynein-based motility.Nat Commun. 2025 Jul 3;16(1):6122. doi: 10.1038/s41467-025-61105-5. Nat Commun. 2025. PMID: 40610402 Free PMC article.
References
-
- Brissette C A, Fives-Taylor P M. Actinobacillus actinomycetemcomitansmay utilize either actin-dependent or actin-independent mechanisms of invasion. Oral Microbiol Immunol. 1998;13:137–142. - PubMed
-
- Burns R G. Analysis of the colchicine-binding site of β-tubulin. FEBS Lett. 1992;297:205–208. - PubMed
-
- DeBrabander M, Nuydens R, Gurts H, Hopkins C R. Dynamic behavior of the transferrin receptor followed in living epidermoid carcinoma (A431) cells with nanovid microscopy. Cell Motil Cytoskeleton. 1988;9:30–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

