Influence of environmental conditions on hydrogen peroxide formation by Streptococcus gordonii
- PMID: 10569775
- PMCID: PMC97067
- DOI: 10.1128/IAI.67.12.6558-6564.1999
Influence of environmental conditions on hydrogen peroxide formation by Streptococcus gordonii
Abstract
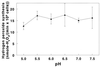
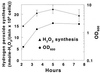
Hydrogen peroxide generated by viridans group streptococci has an antagonistic effect on many bacterial species, including a number of pathogens, in the oral environment. This study examines the influence of a variety of environmental conditions on rates of hydrogen peroxide synthesis by Streptococcus gordonii. Hydrogen peroxide was synthesized at every concentration of glucose and sucrose tested from 10 microM to 1 M, with the highest rates occurring at 0.1 mM sucrose and 1 mM glucose. S. gordonii appeared to have an intracellular store of polysaccharide which supported hydrogen peroxide formation even when the assay buffer contained no carbohydrate. Most heavy metal ions inhibited peroxidogenesis, and anaerobic conditions induced adaptive down-regulation of hydrogen peroxide synthesis; however, peroxidogenesis was generally insensitive to moderate increases in salt concentration, alteration of the mineral content of the assay solution, and changes in pH between 5.0 and 7.5. In contrast, stimulation of peroxidogenesis occurred in 1 mM Mg(2+) and 10 to 50 mM potassium L-lactate. Maximum peroxidogenesis occurred during the mid-logarithmic and late-logarithmic phases of bacterial growth. These bacterial responses may have significant implications for oral ecology and oral health.
Figures


References
-
- Ali R W, Johannessen A C, Dahlen G, Socransky S S, Skaug N. Comparison of the subgingival microbiota of periodontally healthy and diseased adults in northern Cameroon. J Clin Periodontol. 1997;24:830–835. - PubMed
-
- Carlsson J, Edlund M B. Pyruvate oxidase in Streptococcus sanguisunder various growth conditions. Oral Microbiol Immunol. 1987;2:10–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

