The structural basis for the oriented assembly of a TBP/TFB/promoter complex
- PMID: 10570130
- PMCID: PMC24122
- DOI: 10.1073/pnas.96.24.13668
The structural basis for the oriented assembly of a TBP/TFB/promoter complex
Abstract
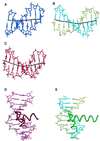
Recently the definition of the metazoan RNA polymerase II and archaeal core promoters has been expanded to include a region immediately upstream of the TATA box called the B recognition element (BRE), so named because eukaryal transcription factor TFIIB and its archaeal orthologue TFB interact with the element in a sequence-specific manner. Here we present the 2.4-A crystal structure of archaeal TBP and the C-terminal core of TFB (TFB(c)) in a complex with an extended TATA-box-containing promoter that provides a detailed picture of the stereospecific interactions between the BRE and a helix-turn-helix motif in the C-terminal cyclin repeat of TFB(c). This interaction is important in determining the level of basal transcription and explicitly defines the direction of transcription.
Figures



References
-
- Hausner W, Wettach J, Hethke C, Thomm M. J Biol Chem. 1996;271:30144–30148. - PubMed
-
- Roeder R G. Trends Biochem Sci. 1996;21:327–335. - PubMed
-
- Baumann P, Qureshi S A, Jackson S P. Trends Genet. 1995;11:279–283. - PubMed
-
- Bell S D, Jackson S P. Cold Spring Harbor Symp Quant Biol. 1998;63:41–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

