Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm
- PMID: 10570136
- PMCID: PMC24128
- DOI: 10.1073/pnas.96.24.13703
Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm
Abstract
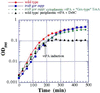
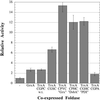
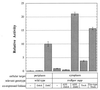
Under physiological conditions, the Escherichia coli cytoplasm is maintained in a reduced state that strongly disfavors the formation of stable disulfide bonds in proteins. However, mutants in which the reduction of both thioredoxins and glutathione is impaired (trxB gor mutants) accumulate oxidized, enzymatically active alkaline phosphatase in the cytoplasm. These mutants grow very poorly in the absence of an exogenous reductant and accumulate extragenic suppressors at a high frequency. One such suppressor strain, FA113, grows almost as rapidly as the wild type in the absence of reductant, exhibits slightly faster kinetics of disulfide bond formation, and has fully induced activity of the transcriptional activator, OxyR. FA113 gave substantially higher yields of properly oxidized proteins compared with wild-type or trxB mutant strains. For polypeptides with very complex patterns of disulfide bonds, such as vtPA and the full-length tPA, the amount of active protein was further enhanced up to 15-fold by co-expression of TrxA (thioredoxin 1) mutants with different redox potentials, or 20-fold by the protein disulfide isomerase, DsbC. Remarkably, higher yields of oxidized, biologically active proteins were obtained by expression in the cytoplasm of E. coli FA113 compared with what could be achieved via secretion into the periplasm of a wild-type strain, even under optimized conditions. These results demonstrate that the cytoplasm can be rendered sufficiently oxidizing to allow efficient formation of native disulfide bonds without compromising cell viability.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

