Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion
- PMID: 10570184
- PMCID: PMC24176
- DOI: 10.1073/pnas.96.24.13978
Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion
Abstract
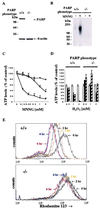
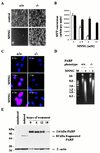
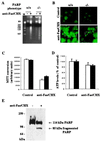
Apoptotic and necrotic cell death are well characterized and are influenced by intracellular ATP levels. Poly(ADP-ribose) polymerase (PARP), a nuclear enzyme activated by DNA strand breaks, physiologically participates in DNA repair. Overactivation of PARP after cellular insults can lead to cell death caused by depletion of the enzyme's substrate beta-nicotinamide adenine dinucleotide and of ATP. In this study, we have differentially elicited apoptosis or necrosis in mouse fibroblasts. Fibroblasts from PARP-deficient (PARP(-/-)) mice are protected from necrotic cell death and ATP depletion but not from apoptotic death. These findings, together with cell death patterns in PARP(-/-) animals receiving other types of insults, indicate that PARP activation is an active trigger of necrosis, whereas other mechanisms mediate apoptosis.
Figures
References
-
- Lautier D, Lagueux J, Thibodeau J, Menard L, Poirier G G. Mol Cell Biochem. 1993;122:171–193. - PubMed
-
- de Murcia G, Schreiber V, Molinete M, Saulier B, Poch O, Masson M, Niedergang C, Menissier de Murcia J. Mol Cell Biochem. 1994;138:15–24. - PubMed
-
- Satoh M S, Lindahl T. Nature (London) 1992;356:356–358. - PubMed
-
- Berger N A. Radiat Res. 1985;101:4–15. - PubMed
-
- Carson D A, Seto S, Wasson D B, Carrera C J. Exp Cell Res. 1986;164:273–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

