Detection of neuritic plaques in Alzheimer's disease by magnetic resonance microscopy
- PMID: 10570201
- PMCID: PMC24193
- DOI: 10.1073/pnas.96.24.14079
Detection of neuritic plaques in Alzheimer's disease by magnetic resonance microscopy
Abstract
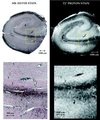
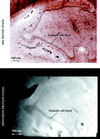
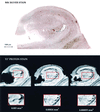
Magnetic resonance microscopy (MRM) theoretically provides the spatial resolution and signal-to-noise ratio needed to resolve neuritic plaques, the neuropathological hallmark of Alzheimer's disease (AD). Two previously unexplored MR contrast parameters, T2* and diffusion, are tested for plaque-specific contrast to noise. Autopsy specimens from nondemented controls (n = 3) and patients with AD (n = 5) were used. Three-dimensional T2* and diffusion MR images with voxel sizes ranging from 3 x 10(-3) mm(3) to 5.9 x 10(-5) mm(3) were acquired. After imaging, specimens were cut and stained with a microwave king silver stain to demonstrate neuritic plaques. From controls, the alveus, fimbria, pyramidal cell layer, hippocampal sulcus, and granule cell layer were detected by either T2* or diffusion contrast. These structures were used as landmarks when correlating MRMs with histological sections. At a voxel resolution of 5.9 x 10(-5) mm(3), neuritic plaques could be detected by T2*. The neuritic plaques emerged as black, spherical elements on T2* MRMs and could be distinguished from vessels only in cross-section when presented in three dimension. Here we provide MR images of neuritic plaques in vitro. The MRM results reported provide a new direction for applying this technology in vivo. Clearly, the ability to detect and follow the early progression of amyloid-positive brain lesions will greatly aid and simplify the many possibilities to intervene pharmacologically in AD.
Figures

References
-
- Johnson G A, Benveniste H, Black R D, Hedlund L W, Maronpot R R, Smith B R. Magn Reson Q. 1993;9:1–30. - PubMed
-
- Johnson G A, Benveniste H, Engelhardt R T, Qui H, Hedlund L W. Ann N Y Acad Sci. 1997;820:139–148. - PubMed
-
- Smith B, Linney E, Huff D, Johnson G. Comp Med Imag Graphics. 1996;20:483–490. - PubMed
-
- Probst A, Langui D, Ulrich J. Brain Pathol. 1991;1:229–239. - PubMed
-
- Esiri M M, Hyman B T, Beyreuther K, Masters C L. In: Greenfield’s Neuropathology. Graham D I, Lantos P L, editors. Vol. 2. London: Arnold; 1997. pp. 153–234.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

