Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid
- PMID: 10570203
- PMCID: PMC24195
- DOI: 10.1073/pnas.96.24.14088
Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid
Abstract
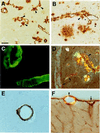
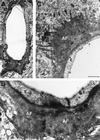
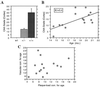
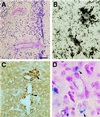
Transgenic mice that overexpress mutant human amyloid precursor protein (APP) exhibit one hallmark of Alzheimer's disease pathology, namely the extracellular deposition of amyloid plaques. Here, we describe significant deposition of amyloid beta (Abeta) in the cerebral vasculature [cerebral amyloid angiopathy (CAA)] in aging APP23 mice that had striking similarities to that observed in human aging and Alzheimer's disease. Amyloid deposition occurred preferentially in arterioles and capillaries and within individual vessels showed a wide heterogeneity (ranging from a thin ring of amyloid in the vessel wall to large plaque-like extrusions into the neuropil). CAA was associated with local neuron loss, synaptic abnormalities, microglial activation, and microhemorrhage. Although several factors may contribute to CAA in humans, the neuronal origin of transgenic APP, high levels of Abeta in cerebrospinal fluid, and regional localization of CAA in APP23 mice suggest transport and drainage pathways rather than local production or blood uptake of Abeta as a primary mechanism underlying cerebrovascular amyloid formation. APP23 mice on an App-null background developed a similar degree of both plaques and CAA, providing further evidence that a neuronal source of APP/Abeta is sufficient to induce cerebrovascular amyloid and associated neurodegeneration.
Figures

References
-
- Vinters H V. Stroke. 1987;18:311–324. - PubMed
-
- Levy E, Carman M D, Fernandez-Madrid I J, Power M D, Lieberburg I, van Duinen S G, Bots G T, Luyendijk W, Frangione B. Science. 1990;248:1124–1126. - PubMed
-
- Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T. J Neurol Sci. 1993;116:135–141. - PubMed
-
- Kawai M, Kalaria R N, Cras P, Siedlak S L, Velasco M E, Shelton E R, Chan H W, Greenberg B D, Perry G. Brain Res. 1993;623:142–146. - PubMed
-
- Walker L C. Brain Res Brain Res Rev. 1997;25:70–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

