Molecular evidence for a new bacteriophage of Borrelia burgdorferi
- PMID: 10572135
- PMCID: PMC103694
- DOI: 10.1128/JB.181.23.7308-7313.1999
Molecular evidence for a new bacteriophage of Borrelia burgdorferi
Abstract
We have recovered a DNase-protected, chloroform-resistant molecule of DNA from the cell-free supernatant of a Borrelia burgdorferi culture. The DNA is a 32-kb double-stranded linear molecule that is derived from the 32-kb circular plasmids (cp32s) of the B. burgdorferi genome. Electron microscopy of samples from which the 32-kb DNA molecule was purified revealed bacteriophage particles. The bacteriophage has a polyhedral head with a diameter of 55 nm and appears to have a simple 100-nm-long tail. The phage is produced constitutively at low levels from growing cultures of some B. burgdorferi strains and is inducible to higher levels with 10 microg of 1-methyl-3-nitroso-nitroguanidine (MNNG) ml(-1). In addition, the prophage can be induced with MNNG from some Borrelia isolates that do not naturally produce phage. We have isolated and partially characterized the phage associated with B. burgdorferi CA-11.2A. To our knowledge, this is the first molecular characterization of a bacteriophage of B. burgdorferi.
Figures
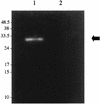
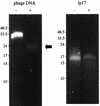



References
-
- Amla D V. Mutagenesis of free and intracellular cyanophage AS-1 by ultraviolet, N-methanyl-N′-nitro-N-nitrosoguanidine and acriflavin. Mutat Res. 1979;59:147–155. - PubMed
-
- Balganesh M, Setlow J K. Prophage induction in Haemophilus influenzae and its relationship to mutation by chemical and physical agents. Mutat Res. 1984;125:15–22. - PubMed
-
- Barbour A G, Garon C F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987;237:409–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

