Overexpression of cysteine-string proteins in Drosophila reveals interactions with syntaxin
- PMID: 10575024
- PMCID: PMC6782430
- DOI: 10.1523/JNEUROSCI.19-23-10270.1999
Overexpression of cysteine-string proteins in Drosophila reveals interactions with syntaxin
Abstract
Cysteine-string proteins (CSPs) are associated with secretory vesicles and critical for regulated neurotransmitter release and peptide exocytosis. At nerve terminals, CSPs have been implicated in the mediation of neurotransmitter exocytosis by modulating presynaptic calcium channels; however, studies of CSPs in peptidergic secretion suggest a direct role in exocytosis independent of calcium transmembrane fluxes. Here we show that the individual expression of various CSP isoforms in Drosophila similarly rescues the loss of evoked neurotransmitter release at csp null mutant motor nerve terminals, suggesting widely overlapping functions for each isoform. Thus, the structural difference of CSP variants may not explain the opposing putative functions of CSP in neurotransmitter and peptide exocytosis. Consistently, the individual overexpression of each CSP isoform in wild-type Drosophila shows similar effects such as impaired viability and interference with wing and eye development. The dominant effects caused by the overexpression of CSP are suppressed by the simultaneous overexpression of syntaxin-1A but not by the coexpression of SNAP-25. Although overexpression of CSP itself has no apparent effect on the synaptic physiology of larval motor nerve terminals, it fully suppresses the decrease of evoked release induced by the overexpression of syntaxin-1A. A direct protein-protein interaction of CSP with syntaxin is further supported by coimmunoprecipitations of syntaxin with CSP and by protein binding assays using recombinant fusion proteins. Together, the genetic and biochemical interactions of CSP and syntaxin-1A suggest that CSP may chaperone or modulate protein-protein interactions of syntaxin-1A with either calcium channels or other components of the regulatory machinery mediating depolarization-dependent neurotransmitter exocytosis.
Figures

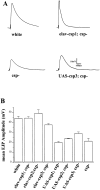
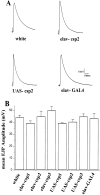
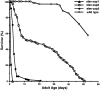

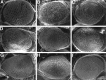

Similar articles
-
Cysteine-string protein increases the calcium sensitivity of neurotransmitter exocytosis in Drosophila.J Neurosci. 2000 Aug 15;20(16):6039-47. doi: 10.1523/JNEUROSCI.20-16-06039.2000. J Neurosci. 2000. PMID: 10934253 Free PMC article.
-
Interactions between proteins implicated in exocytosis and voltage-gated calcium channels.Philos Trans R Soc Lond B Biol Sci. 1999 Feb 28;354(1381):289-97. doi: 10.1098/rstb.1999.0380. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10212477 Free PMC article. Review.
-
The synaptic vesicle protein, cysteine-string protein, is associated with the plasma membrane in 3T3-L1 adipocytes and interacts with syntaxin 4.J Cell Sci. 2001 Jan;114(Pt 2):445-55. doi: 10.1242/jcs.114.2.445. J Cell Sci. 2001. PMID: 11148145
-
Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo.Neuron. 1999 Jul;23(3):593-605. doi: 10.1016/s0896-6273(00)80811-9. Neuron. 1999. PMID: 10433270
-
Cysteine-string protein: the chaperone at the synapse.J Neurochem. 2000 May;74(5):1781-9. doi: 10.1046/j.1471-4159.2000.0741781.x. J Neurochem. 2000. PMID: 10800920 Review.
Cited by
-
Quercetin targets cysteine string protein (CSPalpha) and impairs synaptic transmission.PLoS One. 2010 Jun 10;5(6):e11045. doi: 10.1371/journal.pone.0011045. PLoS One. 2010. PMID: 20548785 Free PMC article.
-
Synaptic tau: A pathological or physiological phenomenon?Acta Neuropathol Commun. 2021 Sep 9;9(1):149. doi: 10.1186/s40478-021-01246-y. Acta Neuropathol Commun. 2021. PMID: 34503576 Free PMC article. Review.
-
Quantitative genomics of locomotor behavior in Drosophila melanogaster.Genome Biol. 2007;8(8):R172. doi: 10.1186/gb-2007-8-8-r172. Genome Biol. 2007. PMID: 17708775 Free PMC article.
-
Phosphorylation-dependent interaction of the synaptic vesicle proteins cysteine string protein and synaptotagmin I.Biochem J. 2002 Jun 1;364(Pt 2):343-7. doi: 10.1042/BJ20020123. Biochem J. 2002. PMID: 11931641 Free PMC article.
-
Regulated Alternative Splicing of Drosophila Dscam2 Is Necessary for Attaining the Appropriate Number of Photoreceptor Synapses.Genetics. 2018 Feb;208(2):717-728. doi: 10.1534/genetics.117.300432. Epub 2017 Dec 5. Genetics. 2018. PMID: 29208630 Free PMC article.
References
-
- Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. - PubMed
-
- Braun J, Wilbanks SM, Scheller RH. The cysteine string secretory vesicle protein activates Hsc70 ATPase. J Biol Chem. 1996;271:25989–25993. - PubMed
-
- Brown H, Larsson O, Branstrom R, Yang SN, Leibiger B, Leibiger I, Fried G, Moede T, Deeney JT, Brown GR, Jacobsson G, Rhodes CJ, Braun JE, Scheller RH, Corkey BE, Berggren PO, Meister B. Cysteine string protein (CSP) is an insulin secretory granule-associated protein regulating beta-cell exocytosis. EMBO J. 1998;17:5048–5058. - PMC - PubMed
-
- Buchner E, Gundersen CB. The DnaJ-like cysteine string protein and exocytotic neurotransmitter release. Trends Neurosci. 1997;20:223–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
