Analysis of the role of heat shock protein (Hsp) molecular chaperones in polyglutamine disease
- PMID: 10575031
- PMCID: PMC6782415
- DOI: 10.1523/JNEUROSCI.19-23-10338.1999
Analysis of the role of heat shock protein (Hsp) molecular chaperones in polyglutamine disease
Abstract
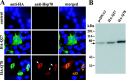
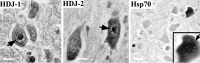
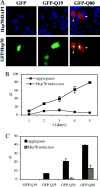
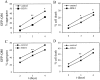
Polyglutamine (polygln) diseases are a group of inherited neurodegenerative disorders characterized by protein misfolding and aggregation. Here, we investigate the role in polygln disease of heat shock proteins (Hsps), the major class of molecular chaperones responsible for modulating protein folding in the cell. In transfected COS7 and PC12 neural cells, we show that Hsp40 and Hsp70 chaperones localize to intranuclear aggregates formed by either mutant ataxin-3, the disease protein in spinocerebellar ataxia type 3/Machado-Joseph disease (SCA3/MJD), or an unrelated green fluorescent protein fusion protein containing expanded polygln. We further demonstrate that expression of expanded polygln protein elicits a stress response in cells as manifested by marked induction of Hsp70. Studies of SCA3/MJD disease brain confirm these findings, showing localization of Hsp40 and, less commonly, Hsp70 chaperones to intranuclear ataxin-3 aggregates. In transfected cells, overexpression of either of two Hsp40 chaperones, the DNAJ protein homologs HDJ-1 and HDJ-2, suppresses aggregation of truncated or full-length mutant ataxin-3. Finally, we extend these studies to a PC12 neural model of polygln toxicity in which we demonstrate that overexpression of HDJ-1 suppresses polygln aggregation with a parallel decrease in toxicity. These results suggest that expanded polygln protein induces a stress response and that specific molecular chaperones may aid the handling of misfolded or aggregated polygln protein in neurons. This study has therapeutic implications because it suggests that efforts to increase chaperone activity may prove beneficial in this class of diseases.
Figures



References
-
- Bukau B, Horwich AL. The Hsp 70 and Hsp 60 chaperone machines. Cell. 1998;92:351–366. - PubMed
-
- Chai Y, Koppenhafer S, Shoesmith S, Perez M, Paulson H. Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Hum Mol Genet. 1999;8:673–682. - PubMed
-
- Cooper J, Schilling G, Peters M, Herring W, Sharp A, Kaminsky Z, Masone J, Khan F, Delanoy M, Borchelt D, Dawson V, Dawson T, Ross C. Truncated N-terminal fragments of huntingtin with expanded glutamine repeats form nuclear and cytoplasmic aggregates in cell culture. Hum Mol Genet. 1998;7:783–790. - PubMed
-
- Cummings CJ, Mancini MA, Antalffy B, DeFranco DB, Orr HT, Zoghbi HY. Chaperone suppression of ataxin-1 aggregation and altered subcellular proteosome localization imply protein misfolding in SCA1. Nat Genet. 1998;19:148–154. - PubMed
-
- Cyr DM, Langer T, Douglas MG. Dna-J like proteins: molecular chaperones and specific regulators of Hsp 70. Trends Biochem Sci. 1994;19:176–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
