The contribution of facilitation of monosynaptic PSPs to dishabituation and sensitization of the Aplysia siphon withdrawal reflex
- PMID: 10575041
- PMCID: PMC6782414
- DOI: 10.1523/JNEUROSCI.19-23-10438.1999
The contribution of facilitation of monosynaptic PSPs to dishabituation and sensitization of the Aplysia siphon withdrawal reflex
Abstract
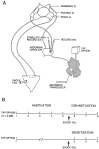
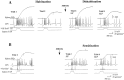
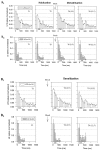
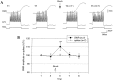
To examine the relationship between synaptic plasticity and learning and memory as directly as possible, we have developed a new simplified preparation for studying the siphon-withdrawal reflex of Aplysia in which it is relatively easy to record synaptic connections between individual identified neurons during simple forms of learning. We estimated that monosynaptic EPSPs from LE siphon sensory neurons to LFS siphon motor neurons mediate approximately one-third of the reflex response measured in this preparation, which corresponds to siphon flaring in the intact animal. To investigate cellular mechanisms contributing to dishabituation and sensitization, we recorded evoked firing of LFS neurons, the siphon withdrawal produced by stimulation of an LFS neuron, the complex PSP in an LFS neuron, and the monosynaptic PSP from an "on-field" or "off-field" LE neuron to an LFS neuron during behavioral training. Unlike the simplified gill-withdrawal preparation (Cohen et al., 1997; Frost et al., 1997), in the siphon-withdrawal preparation we found no qualitative differences between the major cellular mechanisms contributing to dishabituation and sensitization, suggesting that dissociations that have been observed previously may be attributable to transient inhibition that does not occur for this component of the reflex. Furthermore, in the siphon-withdrawal preparation, all of the various cellular measures, including monosynaptic PSPs from either on-field or off-field LE neurons, changed approximately in parallel with changes in the behavior. These results provide the most direct evidence so far available that both dishabituation and sensitization involve multiple mechanisms, including heterosynaptic facilitation of sensory neuron-motor neuron PSPs.
Figures











References
-
- Antonov IN, Osipenko ON, Storozhyk VM. Analysis of heterosynaptic facilitation in identified giant neurons from cerebral ganglion of the pond snail Planorbis corneus. Comp Biochem Physiol. 1991;98C:323–327. - PubMed
-
- Bailey CH, Castellucci VF, Koester J, Kandel ER. Cellular studies of peripheral neurons in siphon skin of Aplysia californica. J Neurophysiol. 1979;42:530–557. - PubMed
-
- Bliss TVP, Collingridge G. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Byrne JH, Castellucci VF, Kandel ER. Receptive fields and response properties of mechanoreceptor neurons innervating siphon skin and mantle shelf of Aplysia. J Neurophysiol. 1974;37:1041–1064. - PubMed
-
- Byrne JH, Castellucci VF, Kandel ER. Contribution of individual mechanoreceptor sensory neurons to defensive gill-withdrawal reflex in Aplysia. J Neurophysiol. 1978;41:418–431. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
