The hamster circadian rhythm system includes nuclei of the subcortical visual shell
- PMID: 10575044
- PMCID: PMC6782426
- DOI: 10.1523/JNEUROSCI.19-23-10482.1999
The hamster circadian rhythm system includes nuclei of the subcortical visual shell
Abstract
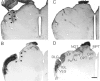
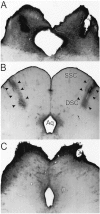
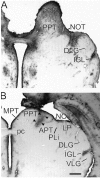
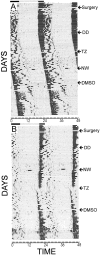
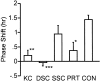
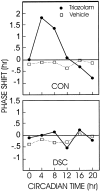
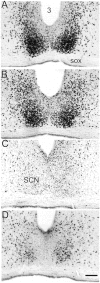
The clock regulating mammalian circadian rhythmicity resides in the suprachiasmatic nucleus. The intergeniculate leaflet, a major component of the subcortical visual system, has been shown to be essential for certain aspects of circadian rhythm regulation. We now report that midbrain visual nuclei afferent to the intergeniculate leaflet are also components of the hamster circadian rhythm system. Loss of connections between the intergeniculate leaflet and visual midbrain or neurotoxic lesions of pretectum or deep superior colliculus (but not of the superficial superior colliculus) blocked phase shifts of the circadian activity rhythm in response to a benzodiazepine injection during the subjective day. Such damage did not disturb phase response to a novel wheel stimulus. The amount of wheel running or open field locomotion were equivalent in lesioned and control groups after benzodiazepine treatment. Electrical stimulation of the deep superior colliculus, without its own effect on circadian rhythm phase, greatly attenuated light-induced phase shifts. Such stimulation was associated with increased FOS protein immunoreactivity in the suprachiasmatic nucleus. The results show that the circadian rhythm system includes the visual midbrain and distinguishes between mechanisms necessary for phase response to benzodiazepine and those for phase response to locomotion in a novel wheel. The results also refute the idea that benzodiazepine-induced phase shifts are the consequence of induced locomotion. Finally, the data provide the first indication that the visual midbrain can modulate circadian rhythm response to light. A variety of environmental stimuli may gain access to the circadian clock mechanism through subcortical nuclei projecting to the intergeniculate leaflet and, via the final common path of the geniculohypothalamic tract, from the leaflet to the suprachiasmatic nucleus.
Figures



Similar articles
-
Intergeniculate leaflet: contributions to photic and non-photic responsiveness of the hamster circadian system.Neuroscience. 2006 Jun 19;140(1):305-20. doi: 10.1016/j.neuroscience.2006.01.050. Epub 2006 Mar 23. Neuroscience. 2006. PMID: 16549274
-
Destruction of serotonergic neurons in the median raphe nucleus blocks circadian rhythm phase shifts to triazolam but not to novel wheel access.J Biol Rhythms. 1998 Dec;13(6):494-505. doi: 10.1177/074873098129000327. J Biol Rhythms. 1998. PMID: 9850010
-
Fos protein expression in the circadian clock is not associated with phase shifts induced by a nonphotic stimulus, triazolam.Neurosci Lett. 1993 Dec 24;164(1-2):203-8. doi: 10.1016/0304-3940(93)90892-o. Neurosci Lett. 1993. PMID: 8152601
-
The circadian visual system.Brain Res Brain Res Rev. 1994 Jan;19(1):102-27. doi: 10.1016/0165-0173(94)90005-1. Brain Res Brain Res Rev. 1994. PMID: 7909471 Review.
-
The circadian visual system, 2005.Brain Res Rev. 2006 Jun;51(1):1-60. doi: 10.1016/j.brainresrev.2005.08.003. Epub 2005 Dec 5. Brain Res Rev. 2006. PMID: 16337005 Review.
Cited by
-
Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus.J Neurosci. 2003 Aug 20;23(20):7451-60. doi: 10.1523/JNEUROSCI.23-20-07451.2003. J Neurosci. 2003. PMID: 12930783 Free PMC article.
-
Acute effects of light on the brain and behavior of diurnal Arvicanthis niloticus and nocturnal Mus musculus.Physiol Behav. 2015 Jan;138:75-86. doi: 10.1016/j.physbeh.2014.09.006. Epub 2014 Oct 28. Physiol Behav. 2015. PMID: 25447482 Free PMC article.
-
Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster.Pharmacol Biochem Behav. 2007 Aug-Sep;87(3):297-305. doi: 10.1016/j.pbb.2007.05.001. Epub 2007 May 10. Pharmacol Biochem Behav. 2007. PMID: 17544066 Free PMC article.
-
Light-induced responses of slow oscillatory neurons of the rat olivary pretectal nucleus.PLoS One. 2012;7(3):e33083. doi: 10.1371/journal.pone.0033083. Epub 2012 Mar 12. PLoS One. 2012. PMID: 22427957 Free PMC article.
-
Retinal projections to the subcortical visual system in congenic albino and pigmented rats.Neuroscience. 2006 Dec;143(3):895-904. doi: 10.1016/j.neuroscience.2006.08.016. Epub 2006 Sep 22. Neuroscience. 2006. PMID: 16996223 Free PMC article.
References
-
- Albers HE, Ferris CF. Neuropeptide Y: role in light-dark entrainment of hamster circadian rhythms. Neurosci Lett. 1984;50:163–168. - PubMed
-
- Albers HE, Ferris CF, Leeman SE, Goldman BD. Avian pancreatic polypeptide phase shifts hamster circadian rhythms when microinjected into the suprachiasmatic region. Science. 1984;223:833–835. - PubMed
-
- Biello SM. Enhanced photic phase shifting after treatment with antiserum to neuropeptide Y. Brain Res. 1995;673:25–29. - PubMed
-
- Biello SM, Mrosovsky N. Circadian phase-shifts induced by chlordiazepoxide without increased locomotor activity. Brain Res. 1993;622:58–62. - PubMed
-
- Biello SM, Harrington ME, Mason R. Geniculo-hypothalamic tract lesions block chlordiazepoxide- induced phase advances in Syrian hamsters. Brain Res. 1991;552:47–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
