How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues
- PMID: 10575046
- PMCID: PMC6782432
- DOI: 10.1523/JNEUROSCI.19-23-10502.1999
How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues
Abstract
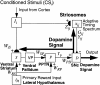
After classically conditioned learning, dopaminergic cells in the substantia nigra pars compacta (SNc) respond immediately to unexpected conditioned stimuli (CS) but omit formerly seen responses to expected unconditioned stimuli, notably rewards. These cells play an important role in reinforcement learning. A neural model explains the key neurophysiological properties of these cells before, during, and after conditioning, as well as related anatomical and neurophysiological data about the pedunculopontine tegmental nucleus (PPTN), lateral hypothalamus, ventral striatum, and striosomes. The model proposes how two parallel learning pathways from limbic cortex to the SNc, one devoted to excitatory conditioning (through the ventral striatum, ventral pallidum, and PPTN) and the other to adaptively timed inhibitory conditioning (through the striosomes), control SNc responses. The excitatory pathway generates CS-induced excitatory SNc dopamine bursts. The inhibitory pathway prevents dopamine bursts in response to predictable reward-related signals. When expected rewards are not received, striosomal inhibition of SNc that is unopposed by excitation results in a phasic drop in dopamine cell activity. The adaptively timed inhibitory learning uses an intracellular spectrum of timed responses that is proposed to be similar to adaptively timed cellular mechanisms in the hippocampus and cerebellum. These mechanisms are proposed to include metabotropic glutamate receptor-mediated Ca(2+) spikes that occur with different delays in striosomal cells. A dopaminergic burst in concert with a Ca(2+) spike is proposed to potentiate inhibitory learning. The model provides a biologically predictive alternative to temporal difference conditioning models and explains substantially more data than alternative models.
Figures




References
-
- Berns G, Sejnowski T. A computational model of how the basal ganglia produce sequences. J Cognit Neurosci. 1998;10:108–121. - PubMed
-
- Berridge K, Robinson T. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. - PubMed
-
- Brog J, Salyapongse A, Deutch A, Zahm D. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. - PubMed
-
- Buonomano DV, Mauk MD. Neural network model of the cerebellum: temporal discrimination and the timing of motor responses. Neural Comput. 1994;6:38–55.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
