Infantile spasms: hypothesis-driven therapy and pilot human infant experiments using corticotropin-releasing hormone receptor antagonists
- PMID: 10575251
- PMCID: PMC3139473
- DOI: 10.1159/000017407
Infantile spasms: hypothesis-driven therapy and pilot human infant experiments using corticotropin-releasing hormone receptor antagonists
Abstract
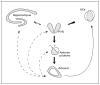
Background and rationale: Infantile spasms (IS) are an age-specific seizure disorder occurring in 1:2,000 infants and associated with mental retardation in approximately 90% of affected individuals. The costs of IS in terms of loss of lifetime productivity and emotional and financial burdens on families are enormous. It is generally agreed that the seizures associated with IS respond poorly to most conventional anticonvulsants. In addition, in the majority of patients, a treatment course with high-dose corticotropin (ACTH) arrests the seizures completely within days, often without recurrence on discontinuation of the hormone. However, the severe side effects of ACTH require development of better treatments for IS. Based on the rapid, all-or-none and irreversible effects of ACTH and on the established physiological actions of this hormone, it was hypothesized that ACTH eliminated IS via an established neuroendocrine feedback mechanism involving suppression of the age-specific endogenous convulsant neuropeptide corticotropin-releasing hormone (CRH). Indeed, IS typically occur in the setting of injury or insult that activate the CNS stress system, of which CRH is a major component. CRH levels may be elevated in the IS brain, and the neuropeptide is known to cause seizures in infant rats, as well as neuronal death in brain regions involved in learning and memory. If 'excess' CRH is involved in the pathogenesis of IS, then blocking CRH receptors should eliminate both seizures and the excitotoxicity of CRH-receptor-rich neurons subserving learning and memory.
Patients and methods: With FDA approval, alpha-helical CRH, a competitive antagonist of the peptide, was given as a phase I trial to 6 infants with IS who have either failed conventional treatment or who have suffered a recurrence. The study was performed at the Clinical Research Center of the Childrens Hospital, Los Angeles. The effects of alpha-helical CRH on autonomic parameters (blood pressure, pulse, temperature, respiration) were determined. In addition, immediate and short-term effects on ACTH and cortisol and on electrolytes and glucose were examined. The potential efficacy of alpha-helical CRH for IS was studied, using clinical diaries and video EEG.
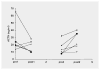
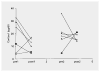
Results: alpha-Helical CRH, a peptide, did not alter autonomic or biochemical parameters. Blocking peripheral CRH receptors was evident from a transient reduction in plasma ACTH and cortisol. No evidence for the compound's penetration of the blood-brain barrier was found, since no central effects on arousal, activity or seizures and EEG patterns were observed. In addition, a striking resistance of the patients' plasma ACTH to the second infusion of alpha-helical CRH was noted.
Conclusions: Peptide analogs of CRH do not cross the blood-brain barrier, and their effects on peripheral stress hormones are transient and benign. Nonpeptide compouds that reach CNS receptors are required to test the hypothesis that blocking CRH receptors may ameliorate IS and its cognitive consequences.
Copyright 1999 S. Karger AG, Basel
Figures
Similar articles
-
How do the many etiologies of West syndrome lead to excitability and seizures? The corticotropin releasing hormone excess hypothesis.Brain Dev. 2001 Nov;23(7):533-8. doi: 10.1016/s0387-7604(01)00312-6. Brain Dev. 2001. PMID: 11701250 Free PMC article. Review.
-
ACTH treatment of infantile spasms: mechanisms of its effects in modulation of neuronal excitability.Int Rev Neurobiol. 2002;49:185-97. doi: 10.1016/s0074-7742(02)49013-7. Int Rev Neurobiol. 2002. PMID: 12040892 Free PMC article. Review.
-
ACTH does not control neonatal seizures induced by administration of exogenous corticotropin-releasing hormone.Epilepsia. 1995 Feb;36(2):174-8. doi: 10.1111/j.1528-1157.1995.tb00977.x. Epilepsia. 1995. PMID: 7821275 Free PMC article.
-
Inhibition of pituitary-adrenal secretion by a corticotropin releasing hormone antagonist in humans.Mol Psychiatry. 1996 Sep;1(4):320-4. Mol Psychiatry. 1996. PMID: 9118358 Free PMC article. Clinical Trial.
-
Pathophysiology of massive infantile spasms: perspective on the putative role of the brain adrenal axis.Ann Neurol. 1993 Mar;33(3):231-6. doi: 10.1002/ana.410330302. Ann Neurol. 1993. PMID: 8388675 Free PMC article. Review.
Cited by
-
Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression.Ann Neurol. 2001 Mar;49(3):304-12. Ann Neurol. 2001. PMID: 11261504 Free PMC article.
-
Pathogenesis and new candidate treatments for infantile spasms and early life epileptic encephalopathies: A view from preclinical studies.Neurobiol Dis. 2015 Jul;79:135-49. doi: 10.1016/j.nbd.2015.04.015. Epub 2015 May 9. Neurobiol Dis. 2015. PMID: 25968935 Free PMC article. Review.
-
How do the many etiologies of West syndrome lead to excitability and seizures? The corticotropin releasing hormone excess hypothesis.Brain Dev. 2001 Nov;23(7):533-8. doi: 10.1016/s0387-7604(01)00312-6. Brain Dev. 2001. PMID: 11701250 Free PMC article. Review.
-
The Oral Findings and Dental Management of Patients with West Syndrome: A Case Series and Literature Review.J Clin Med. 2025 Apr 6;14(7):2494. doi: 10.3390/jcm14072494. J Clin Med. 2025. PMID: 40217943 Free PMC article.
-
ACTH treatment of infantile spasms: mechanisms of its effects in modulation of neuronal excitability.Int Rev Neurobiol. 2002;49:185-97. doi: 10.1016/s0074-7742(02)49013-7. Int Rev Neurobiol. 2002. PMID: 12040892 Free PMC article. Review.
References
-
- Aicardi J. Infantile spasms and related syndromes. In: Aicardi J, editor. Epilepsy in Children. New York: Raven Press; 1986. pp. 17–38.
-
- Sorel L, Dusaucy-Bauloye E. A propos de 21 cas d’hypsarythmia de Gibbs: son traitement spectaculaire par l’ACTH. Acta Neurol Psychiatr Belg. 1958;58:130–141. - PubMed
-
- Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J. Chemical and biological characterization of corticotropin releasing factor. Rec Prog Horm Res. 1983;39:245–270. - PubMed

