Etanercept: therapeutic use in patients with rheumatoid arthritis
- PMID: 10577976
- PMCID: PMC1766576
- DOI: 10.1136/ard.58.2008.i65
Etanercept: therapeutic use in patients with rheumatoid arthritis
Abstract
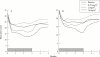
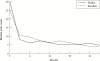
Tumour necrosis factor (TNF) plays a central part in the pathophysiology of rheumatoid arthritis (RA). TNF initiates signal transduction by interacting with surface bound TNF receptors. Soluble tumour necrosis factor receptors (sTNFRs) act as natural inhibitors of TNF activity. Etanercept, recombinant p75 sTNFR:Fc fusion protein, has received approval from the US Food and Drug Administration for patients with RA and juvenile RA (JRA) who have failed treatment with at least one other drug. Etanercept has demonstrated excellent safety and efficacy in large scale, randomised, double blind, placebo controlled trials of patients with RA and JRA who are refractory to other disease modifying anti-rheumatic drugs. The therapeutic effects mediated by etanercept are rapid and sustained. Combining etanercept with methotrexate was found to be safe and more effective than treatment with methotrexate alone in the treatment of RA. These clinical findings demonstrate that etanercept can result in symptomatic improvement in patients with RA and JRA. Etanercept is an important new addition to the treatment of these diseases.
Figures
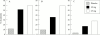
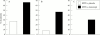
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

