Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis
- PMID: 10579713
- PMCID: PMC2169345
- DOI: 10.1083/jcb.147.5.921
Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis
Abstract
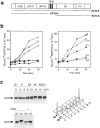
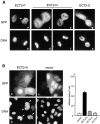
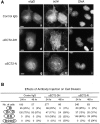
Animal cells divide into two daughter cells by the formation of an actomyosin-based contractile ring through a process called cytokinesis. Although many of the structural elements of cytokinesis have been identified, little is known about the signaling pathways and molecular mechanisms underlying this process. Here we show that the human ECT2 is involved in the regulation of cytokinesis. ECT2 catalyzes guanine nucleotide exchange on the small GTPases, RhoA, Rac1, and Cdc42. ECT2 is phosphorylated during G2 and M phases, and phosphorylation is required for its exchange activity. Unlike other known guanine nucleotide exchange factors for Rho GTPases, ECT2 exhibits nuclear localization in interphase, spreads throughout the cytoplasm in prometaphase, and is condensed in the midbody during cytokinesis. Expression of an ECT2 derivative, containing the NH(2)-terminal domain required for the midbody localization but lacking the COOH-terminal catalytic domain, strongly inhibits cytokinesis. Moreover, microinjection of affinity-purified anti-ECT2 antibody into interphase cells also inhibits cytokinesis. These results suggest that ECT2 is an important link between the cell cycle machinery and Rho signaling pathways involved in the regulation of cell division.
Figures

Similar articles
-
Rho exchange factor ECT2 is induced by growth factors and regulates cytokinesis through the N-terminal cell cycle regulator-related domains.J Cell Biochem. 2003 Nov 1;90(4):819-36. doi: 10.1002/jcb.10688. J Cell Biochem. 2003. PMID: 14587037
-
Deregulation and mislocalization of the cytokinesis regulator ECT2 activate the Rho signaling pathways leading to malignant transformation.J Biol Chem. 2004 Feb 20;279(8):7169-79. doi: 10.1074/jbc.M306725200. Epub 2003 Nov 25. J Biol Chem. 2004. PMID: 14645260
-
Potential roles of the nucleotide exchange factor ECT2 and Cdc42 GTPase in spindle assembly in Xenopus egg cell-free extracts.J Cell Biochem. 2003 Dec 1;90(5):892-900. doi: 10.1002/jcb.10750. J Cell Biochem. 2003. PMID: 14624449
-
Rho GTPases in animal cell mitosis.Curr Opin Cell Biol. 2006 Apr;18(2):199-205. doi: 10.1016/j.ceb.2006.02.002. Epub 2006 Feb 17. Curr Opin Cell Biol. 2006. PMID: 16487696 Review.
-
Rho GTPases as regulators of mitosis and cytokinesis in mammalian cells.Small GTPases. 2014;5:e29770. doi: 10.4161/sgtp.29770. Epub 2014 Jul 2. Small GTPases. 2014. PMID: 24988197 Free PMC article. Review.
Cited by
-
An anillin-Ect2 complex stabilizes central spindle microtubules at the cortex during cytokinesis.PLoS One. 2012;7(4):e34888. doi: 10.1371/journal.pone.0034888. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514687 Free PMC article.
-
KIF14 and citron kinase act together to promote efficient cytokinesis.J Cell Biol. 2006 Jan 30;172(3):363-72. doi: 10.1083/jcb.200511061. Epub 2006 Jan 23. J Cell Biol. 2006. PMID: 16431929 Free PMC article.
-
Cytokinesis in eukaryotes.Microbiol Mol Biol Rev. 2002 Jun;66(2):155-78. doi: 10.1128/MMBR.66.2.155-178.2002. Microbiol Mol Biol Rev. 2002. PMID: 12040122 Free PMC article. Review.
-
The checkpoint-dependent nuclear accumulation of Rho1p exchange factor Rgf1p is important for tolerance to chronic replication stress.Mol Biol Cell. 2014 Apr;25(7):1137-50. doi: 10.1091/mbc.E13-11-0689. Epub 2014 Jan 29. Mol Biol Cell. 2014. PMID: 24478458 Free PMC article.
-
A GAP that Divides.F1000Res. 2017 Oct 2;6:1788. doi: 10.12688/f1000research.12064.1. eCollection 2017. F1000Res. 2017. PMID: 29043078 Free PMC article. Review.
References
-
- Bork P., Hofmann K., Bucher P., Neuwald A., Altschul S., Koonin E. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997;11:68–76. - PubMed
-
- Chant J., Pringle J.R. Budding and cell polarity in Saccharomyces cerevisiae . Curr. Opin. Genet. Dev. 1991;1:342–350. - PubMed
-
- Drechsel D.N., Hyman A.A., Hall A., Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr. Biol. 1997;7:12–23. - PubMed
-
- Goto H., Kosako H., Tanabe K., Yanagida M., Sakurai M., Amano M., Kaibuchi K., Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J. Biol. Chem. 1998;273:11728–11736. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

